
ij����С��������ͼװ�ý����Ҵ��Ĵ�����ʵ�鲢��ȡ��ȩ��ͼ������̨��װ�ü���ȥ���ֺ��߱�ʾ�齺�ܡ�����д���пհף�
��1����װ�ó��������¶�Ϊ70��80���ˮԡ�У�Ŀ����________________________��
��2��ʵ��ʱ���ȼ��Ȳ��������еĶ�����˿��Լ1���Ӻ�����������ʱͭ��˿���ʺ���״̬�����Ѿƾ��Ƴ��ߣ�����һ���Ĺ����ٶȣ�ͭ˿�ܳ�ʱ�䱣�ֺ���ֱ��ʵ�������
��3���Ҵ��Ĵ�������Ӧ�� ��Ӧ������ȡ������ȡ������÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4����ʵ������п��ƹ������ٶȺ���Ҫ:
�ٿ��ƹ����ٶȵķ����� ��
���������ٶȹ��췴Ӧ��ֹͣ,ԭ��: ��
���������ٶȹ�����ӦҲ��ֹͣ,ԭ��: ��
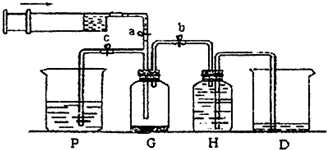
��5�����Թܶ�����ˮ���ղ����Ҫ�ڵ����ҡ���֮�������װ�ã������ӷ����ǣ�����װ���е��ܴ��ţ����ҽ�_____________��______________�ӱ���
��1���ʵ��ӿ������Ҵ����������ʣ����ƽ�ȵ��Ҵ�����
��3������ ��2CH3CH2OH��O2 2CH3CHO��2H2O
2CH3CHO��2H2O
��4���ٿ��Ƽ��е�λʱ����������
�ڴ��߹������������Ա�֤��Ӧ�����¶�
�۷�Ӧ����̫�٣����ܴﵽ��Ӧ��Ҫ�¶�
��5��b �� a
���������������1����װ�ó��������¶�Ϊ70��80���ˮԡ�У��Ҵ��ķе�Ϊ78�棬�����ܹ��ʵ��ӿ������Ҵ����������ʣ����ƽ�ȵ��Ҵ���������3���Ҵ��Ĵ�������Ӧ�Ƿ��ȷ�Ӧ���䷴Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH��O2 2CH3CHO��2H2O����4����ʵ������п��ƹ������ٶȺ���Ҫ:����ͨ�����Ƽ��е�λʱ���������������ƹ����ٶȵķ������������ٶȹ��췴Ӧ��ֹͣ,ԭ���Ǵ��߹������������Ա�֤��Ӧ�����¶ȣ��������ٶȹ�����ӦҲ��ֹͣ,ԭ���Ƿ�Ӧ����̫�٣����ܴﵽ��Ӧ��Ҫ�¶ȣ���5�����Թܶ�����ˮ���ղ����Ҫ�ڵ����ҡ���֮�������װ�ã���װ�õ�����Ϊ��ֹ����������Ӧ�ü��ҽ���b�ӿڴ���a�ӱ���
2CH3CHO��2H2O����4����ʵ������п��ƹ������ٶȺ���Ҫ:����ͨ�����Ƽ��е�λʱ���������������ƹ����ٶȵķ������������ٶȹ��췴Ӧ��ֹͣ,ԭ���Ǵ��߹������������Ա�֤��Ӧ�����¶ȣ��������ٶȹ�����ӦҲ��ֹͣ,ԭ���Ƿ�Ӧ����̫�٣����ܴﵽ��Ӧ��Ҫ�¶ȣ���5�����Թܶ�����ˮ���ղ����Ҫ�ڵ����ҡ���֮�������װ�ã���װ�õ�����Ϊ��ֹ����������Ӧ�ü��ҽ���b�ӿڴ���a�ӱ���
���㣺��������ʵ��
���������⿼���˴�������ʵ�飬��ʵ������ѧ�ľ���ʵ��֮һ����������ڶԸ�ʵ�������������������������ѧ���ķ��������������ѶȽϴ�


 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д� С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012��ɽ���ij�ݷ��ʵ����и߶�������ģ����Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��ÿ��2�֣���18�֣�
��һ��ʵ��������ͼװ����ȡ�����屽������д���пհס�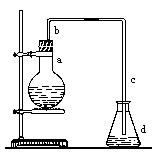
��1������ƿa��װ���Լ��� ��
��2����ֱ����b�����ã� ��
��3�������������c���¿ڿɷ��û��Һ���У� ����ɡ��� ����
��4����Ӧ��Ϻ�����ƿd�еμ�AgNO3��Һ���йط�Ӧ�����ӷ���ʽ�� ��
������ij����С��������ͼװ�ý����Ҵ��Ĵ�����ʵ�鲢��ȡ��ȩ��ͼ������̨��װ������ȥ���ֺ��߱�ʾ�齺�ܡ�����д���пհף�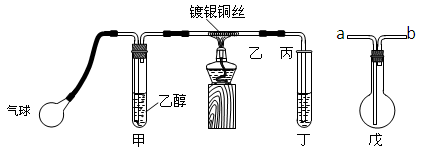
��1����װ�ó�������70��80���ˮԡ�У�Ŀ����
��2��ʵ��ʱ���ȼ��Ȳ��������еĶ���ͭ˿��Լ1���Ӻ�����������ʱͭ˿���ʺ���״̬�����Ѿƾ��Ƴ��ߣ�����һ���Ĺ����ٶȣ�ͭ˿�ܳ�ʱ�䱣�ֺ���ֱ��ʵ�������
�Ҵ��Ĵ�������Ӧ��________��Ӧ������ȡ������ȡ�����
�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3�����Թܶ�����ˮ���ղ����Ҫ�ڵ����ҡ���֮�������װ�ã������ӷ�����
������װ���е��ܴ��ţ����ҽ� ��_______�ӱ���
�����ﲻ��ˮ���ն���ֱ����ȴ��Ӧ���Թܶ����� _____ �С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�����п�����и�һ��ѧ��ѧ�ڳ�����ѧ�Ծ����������� ���ͣ������
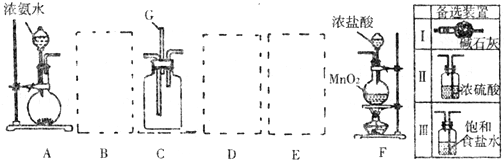
ij����С��������ͼ��ʾװ����ȡ�������ṩ���Լ��У�Ũ���ᡢ����ʳ��ˮ������������Һ��������ع��塣��Ӧ�Ļ�ѧ����ʽΪ��
2KMnO4��+ 16HCl��Ũ���� 2KCl + 2MnCl2��+ 5Cl2��+ 8H2O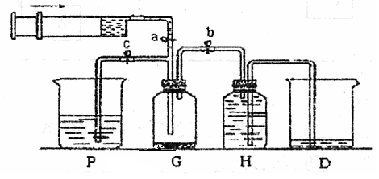
�Իش��������⣺
��1���ڷ�Ӧ2KMnO4 + 16HCl��Ũ���� 2KCl + 2MnCl2 + 5Cl2��+ 8H2O�У�HCl���ֵ�����Ϊ________��_________.�����ɵ�Cl2�����5.6L����״���£�,��ת�Ƶĵ��ӵĸ���________(����٤��������NA����ʾ)
��2��װ��H��ʢ�ŵ��Լ��� ��װ��P��ʢ�ŵ��Լ��� ��
��3��β������ʱ�رյ��ɼ�a�͵��ɼ� �����ɼ� ��
��4������β��ʱ��������Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��������ѵ���и߶���ѧ�ڵ����ο��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��ÿ��2�֣���18�֣�
��һ��ʵ��������ͼװ����ȡ�����屽������д���пհס�
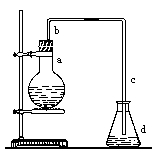
��1������ƿa��װ���Լ��� ��
��2����ֱ����b�����ã� ��
��3�������������c���¿ڿɷ��û��Һ���У� ����ɡ��� ������
��4����Ӧ��Ϻ�����ƿd�еμ�AgNO3��Һ���йط�Ӧ�����ӷ���ʽ�� ��
������ij����С��������ͼװ�ý����Ҵ��Ĵ�����ʵ�鲢��ȡ��ȩ��ͼ������̨��װ������ȥ���ֺ��߱�ʾ�齺�ܡ�����д���пհף�
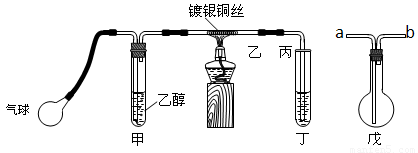
��1����װ�ó�������70��80���ˮԡ�У�Ŀ���� ��
��2��ʵ��ʱ���ȼ��Ȳ��������еĶ���ͭ˿��Լ1���Ӻ�����������ʱͭ˿���ʺ���״̬�����Ѿƾ��Ƴ��ߣ�����һ���Ĺ����ٶȣ�ͭ˿�ܳ�ʱ�䱣�ֺ���ֱ��ʵ�������
�Ҵ��Ĵ�������Ӧ��________��Ӧ������ȡ������ȡ�����
�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3�����Թܶ�����ˮ���ղ����Ҫ�ڵ����ҡ���֮�������װ�ã������ӷ�����
������װ���е��ܴ��ţ����ҽ� ��_______�ӱ���
�����ﲻ��ˮ���ն���ֱ����ȴ��Ӧ���Թܶ����� _____ �С�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com