
X”¢Y”¢Z”¢W”¢RŹĒŌŖĖŲÖÜĘŚ±ķĒ°ĖÄÖÜĘŚÖŠµÄ³£¼ūŌŖĖŲ,ĘäĻą¹ŲŠÅĻ¢ČēĻĀ±ķ:
| ŌŖĖŲ | Ļą¹ŲŠÅĻ¢ |
| X | ×é³Éµ°°×ÖŹµÄ»ł“”ŌŖĖŲ,Ęä×īøßÕż»ÆŗĻ¼ŪÓė×īµĶøŗ»ÆŗĻ¼ŪµÄ“śŹżŗĶĪŖ2 |
| Y | µŲæĒÖŠŗ¬Įæ×īøßµÄŌŖĖŲ |
| Z | “ęŌŚÖŹĮæŹżĪŖ23,ÖŠ×ÓŹżĪŖ11µÄŗĖĖŲ |
| W | Éś»īÖŠ“óĮæŹ¹ÓĆĘäŗĻ½šÖĘĘ·,¹¤ŅµÉĻæÉÓƵē½āČŪČŚŃõ»ÆĪļµÄ·½·ØÖʱøĘ䵄֏ |
| R | ÓŠ¶ąÖÖ»ÆŗĻ¼Ū,Ęä°×É«ĒāŃõ»ÆĪļŌŚæÕĘųÖŠ»įŃøĖŁ±ä³É»ŅĀĢÉ«,×īŗó±ä³ÉŗģŗÖÉ« |
 WŗĻ½š(Z17W12)ŹĒŅ»ÖÖĒ±ŌŚµÄÖüĒā²ÄĮĻ,ÓÉZ”¢Wµ„ÖŹŌŚŅ»¶ØĢõ¼žĻĀČŪĮ¶¶ų³É”£øĆŗĻ½šŌŚŅ»¶ØĢõ¼žĻĀĶźČ«ĪüĒāµÄ·“Ó¦·½³ĢŹ½ĪŖ:Z17W12+17H2
WŗĻ½š(Z17W12)ŹĒŅ»ÖÖĒ±ŌŚµÄÖüĒā²ÄĮĻ,ÓÉZ”¢Wµ„ÖŹŌŚŅ»¶ØĢõ¼žĻĀČŪĮ¶¶ų³É”£øĆŗĻ½šŌŚŅ»¶ØĢõ¼žĻĀĶźČ«ĪüĒāµÄ·“Ó¦·½³ĢŹ½ĪŖ:Z17W12+17H2 17ZH2+12W,µĆµ½µÄ»ģŗĻĪļQ(17ZH2+12W)ŌŚ6.0 mol”¤L-1HClČÜŅŗÖŠÄÜĶźČ«ŹĶ·Å³öH2”£1 mol Z17W12ĶźČ«ĪüĒāŗóµĆµ½µÄ»ģŗĻĪļQÓėÉĻŹöŃĪĖįĶźČ«·“Ó¦,ŹĶ·Å³öH2µÄĪļÖŹµÄĮæĪŖ ”£
17ZH2+12W,µĆµ½µÄ»ģŗĻĪļQ(17ZH2+12W)ŌŚ6.0 mol”¤L-1HClČÜŅŗÖŠÄÜĶźČ«ŹĶ·Å³öH2”£1 mol Z17W12ĶźČ«ĪüĒāŗóµĆµ½µÄ»ģŗĻĪļQÓėÉĻŹöŃĪĖįĶźČ«·“Ó¦,ŹĶ·Å³öH2µÄĪļÖŹµÄĮæĪŖ ”£  ¾ŁŅ»·“Čżµ„ŌŖĶ¬²½¹ż¹Ų¾ķĻµĮŠ“š°ø
¾ŁŅ»·“Čżµ„ŌŖĶ¬²½¹ż¹Ų¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©
M”¢N”¢O”¢P”¢QŹĒŌŖĖŲÖÜĘŚ±ķÖŠŌ×ÓŠņŹżŅĄ“ĪµŻŌöµÄĒ°ĖÄÖÜĘŚŌŖĖŲ”£MŌ×Ó×īĶā²ćµē×ÓŹżĪŖÄŚ²ćµē×ÓŹżµÄ3±¶£»NµÄŃęÉ«·“Ó¦³Ź»ĘÉ«£»OµÄĒā»ÆĪļŹĒŅ»ÖÖĒæĖį£¬ĘäÅØČÜŅŗæÉÓėM”¢QµÄ»ÆŗĻĪļ·“Ӧɜ³ÉOµÄµ„ÖŹ£»PŹĒŅ»ÖÖ½šŹōŌŖĖŲ£¬Ę仳Ģ¬Ō×ÓÖŠÓŠ6øöĪ“³É¶Ōµē×Ó”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŌŖĖŲQµÄĆū³ĘĪŖ__________£¬PµÄ»łĢ¬Ō×Ó¼Ū²ćµē×ÓÅŲ¼Ź½ĪŖ__________ ”£
£Ø2£©OµÄĒā»ÆĪļµÄ·Šµć±ČĘäÉĻŅ»ÖÜĘŚĶ¬×åŌŖĖŲµÄĒā»ÆĪļµĶ£¬ŹĒŅņĪŖ__________________________.
£Ø3£©M”¢OµēøŗŠŌ“óŠ”Ė³ŠņŹĒ__________£ØÓĆŌŖĖŲ·ūŗűķŹ¾£©£¬OµÄ×īøß¼Ūŗ¬ŃõĖįøłµÄæռ乹ŠĶĪŖ__________£¬ĘäÖŠŠÄŌ×ÓµÄŌÓ»ÆĄąŠĶĪŖ___________”£
£Ø4£©M”¢NŠĪ³ÉµÄ»ÆŗĻĪļµÄ¾§°ūČēĶ¼ĖłŹ¾£¬øĆ¾§°ūµÄ»ÆѧŹ½ĪŖ__________ £¬ĘäÖŠMĄė×ÓµÄÅäĪ»ŹżĪŖ__________£¬øĆ¾§ĢåĄąŠĶĪŖ___________”£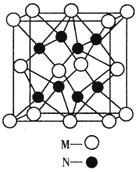
£Ø5£©øĆ¾§°ūµÄ±ß³¤ĪŖa”Į10 cm£¬Ōņ¾ąĄė×ī½üµÄM”¢NĄė×Ó¼äµÄ¾ąĄėĪŖ
cm£¬Ōņ¾ąĄė×ī½üµÄM”¢NĄė×Ó¼äµÄ¾ąĄėĪŖ
__________cmӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
VA×åµÄµŖ”¢Į×”¢Éé(As)µČŌŖĖŲµÄ»ÆŗĻĪļŌŚæĘŃŠŗĶÉś²śÖŠÓŠŠķ¶ąÖŲŅŖÓĆĶ¾£¬Ēė»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©ÉéµÄ»łĢ¬Ō×ӵĵē×ÓÅŲ¼Ź½ĪŖ ”£
£Ø2£©N”¢P”¢AsŌ×ӵĵŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņĪŖ ”£
£Ø3£©NH3µÄ·Šµć±ČPH3øߣ¬ŌŅņŹĒ £»PO43£Ąė×ÓµÄĮ¢Ģå¹¹ŠĶĪŖ ”£
£Ø4£©PH3·Ö×ÓÖŠPŌ×Ó²ÉÓĆ ŌӻƔ£
£Ø5£©H3AsO4ŗĶH3AsO3ŹĒÉéµÄĮ½ÖÖŗ¬ŃõĖį,Ēėøł¾Ż½į¹¹ÓėŠŌÖŹµÄ¹ŲĻµ£¬½āŹĶH3AsO4±ČH3AsO3ĖįŠŌĒæµÄŌŅņ ”£
£Ø6£© CuCl2Óė°±Ė®·“Ó¦æÉŠĪ³ÉÅäŗĻĪļ[Cu(NH3)4]Cl2£¬1moløĆÅäŗĻĪļÖŠŗ¬ÓŠ¦Ņ¼üµÄŹżÄæĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
MŌ×ÓŌŚµŚ¶žÄܲćÉĻÖ»ÓŠŅ»øöæÕ¹ģµĄ£¬ŌņMŹĒ £»Ęäµē×ÓÅŲ¼Ź½ĪŖ £»RŌ×ÓµÄ3p¹ģµĄÉĻÖ»ÓŠŅ»øöĪ“³É¶Ōµē×Ó£¬ŌņRŌ×ÓæÉÄÜŹĒ ”¢ £»YŌ×ÓµÄŗĖµēŗÉŹżĪŖ33£¬ĘäĶāĪ§µē×ÓÅŲ¼ŹĒ £¬ĘäŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒ £¬ŹĒŹōÓŚ ĒųµÄŌŖĖŲ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
X”¢YŗĶZ¾łĪŖ¶ĢÖÜĘŚŌŖĖŲ£¬Ō×ÓŠņŹżŅĄ“ĪŌö“ó£¬XµÄµ„ÖŹĪŖĆܶČ×īŠ”µÄĘųĢ壬YŌ×Ó×īĶā²ćµē×ÓŹżŹĒĘäÖÜĘŚŹżµÄČż±¶£¬ZÓėXŌ×Ó×ī“¦²ćµē×ÓŹżĻąĶ¬”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©X”¢YŗĶZµÄŌŖĖŲ·ūŗÅ·Ö±šĪŖ ”¢ ”¢ ”£
£Ø2£©ÓÉÉĻŹöŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļÖŠ£¬¼Čŗ¬ÓŠ¹²¼Ū¼üÓÖŗ¬ÓŠĄė×Ó¼üµÄÓŠ ”¢ ”£
£Ø3£©XŗĶY×é³ÉµÄ»ÆŗĻĪļÖŠ£¬¼Čŗ¬ÓŠ¼«ŠŌ¹²¼Ū¼üÓÖŗ¬ÓŠ·Ē¼«ŠŌ¹²¼Ū¼üµÄŹĒ ”£“Ė»ÆŗĻĪļŌŚĖįŠŌĢõ¼žĻĀÓėøßĆĢĖį¼Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £»“Ė»ÆŗĻĪļ»¹æɽ«¼īŠŌ¹¤Ņµ·ĻĖ®ÖŠµÄCN-Ńõ»ÆĪŖĢ¼ĖįŃĪŗĶ°±£¬ĻąÓ¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖX”¢Y”¢Z”¢WĖÄÖÖŌŖĖŲŹĒŌŖĖŲÖÜĘŚ±ķÖŠĮ¬ŠųČżøö²»Ķ¬¶ĢÖÜĘŚµÄŌŖĖŲ£¬ĒŅŌ×ÓŠņŹżŅĄ“ĪŌö“ó”£X”¢WĶ¬Ö÷×壬Y”¢ZĪŖĶ¬ÖÜĘŚµÄĻąĮŚŌŖĖŲ”£WŌ×ÓµÄÖŹ×ÓŹżµČÓŚY”¢ZŌ×Ó×īĶā²ćµē×ÓŹżÖ®ŗĶ”£YµÄĒā»ÆĪļ·Ö×ÓÖŠÓŠ3øö¹²¼Ū¼ü”£ZŌ×Ó×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµē×ÓŹżµÄ3±¶”£ŹŌĶʶĻ£ŗ
£Ø1£©X”¢Y”¢Z”¢WĖÄÖÖŌŖĖŲµÄ·ūŗÅ£ŗ
X ӢY ӢZ ӢW
£Ø2£©ÓÉŅŌÉĻŌŖĖŲÖŠµÄĮ½ÖÖŌŖĖŲ×é³ÉµÄÄÜČÜÓŚĖ®ĒŅĖ®ČÜŅŗČõĻŌ¼īŠŌµÄ»ÆŗĻĪļµÄµē×ÓŹ½ĪŖ ”£
£Ø3£©ÓÉX”¢Y”¢ZĖłŠĪ³ÉµÄĄė×Ó»ÆŗĻĪļŹĒ £¬ĖüÓėWµÄ×īøßŃõ»ÆĪļµÄĖ®»ÆĪļµÄÅØČÜŅŗ¼ÓČČŹ±·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ńõ»ÆĀĮ(Al2O3) ŗĶµŖ»Æ¹č£ØSi3N4£©ŹĒÓÅĮ¼µÄøßĪĀ½į¹¹ĢÕ“É£¬ŌŚ¹¤ŅµÉś²śŗĶæĘ¼¼ĮģÓņÓŠÖŲŅŖÓĆĶ¾”£
£Ø1£©AlÓėNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø2£©ĻĀĮŠŹµŃéÄÜ±Č½ĻĆ¾ŗĶĀĮµÄ½šŹōŠŌĒæČõµÄŹĒ £ØĢīŠņŗÅ£©”£
a£®²ā¶ØĆ¾ŗĶĀĮµÄµ¼µēŠŌĒæČõ
b£®²ā¶ØµČĪļÖŹµÄĮæÅØ¶ČµÄAl2(SO4)3ŗĶMgSO4ČÜŅŗµÄpH
c£®Ļņ0.1 mol/LAlCl3ŗĶ0.1 mol/L MgCl2ÖŠ¼Ó¹żĮæNaOHČÜŅŗ
£Ø3£©ĀĮČČ·ØŹĒ³£ÓĆµÄ½šŹōŅ±Į¶·½·ØÖ®Ņ»”£
ŅŃÖŖ£ŗ4Al (s)+3O2(g) =2Al2O3(s) ¦¤H1 =" -3352" kJ/mol
Mn(s)+ O2(g) =MnO2 (s) ¦¤H2 =" -521" kJ/mol
AlÓėMnO2·“Ó¦Ņ±Į¶½šŹōMnµÄČČ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø4£©µŖ»Æ¹čæ¹øÆŹ“ÄÜĮ¦ŗÜĒ棬µ«Ņ×±»Ēā·śĖįøÆŹ“£¬µŖ»Æ¹čÓėĒā·śĖį·“Ӧɜ³ÉĖÄ·ś»Æ¹čŗĶŅ»ÖÖļ§ŃĪ£¬Ęä·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø5£©¹¤ŅµÉĻÓĆ»ÆѧĘųĻą³Į»ż·ØÖʱøµŖ»Æ¹č£¬Ęä·“Ó¦ČēĻĀ£ŗ3SiCl4(g) + 2N2(g) + 6H2(g) Si3N4(s) + 12HCl(g) ”÷H£¼0
Si3N4(s) + 12HCl(g) ”÷H£¼0
ijĪĀ¶ČŗĶŃ¹ĒæĢõ¼žĻĀ£¬·Ö±š½«0.3mol SiCl4(g)”¢0.2mol N2(g)”¢0.6mol H2(g)³äČė2LĆܱÕČŻĘ÷ÄŚ£¬½ųŠŠÉĻŹö·“Ó¦£¬5min“ļµ½Ę½ŗāדĢ¬£¬ĖłµĆSi3N4(s)µÄÖŹĮæŹĒ5.60g”£
¢ŁH2µÄĘ½¾ł·“Ó¦ĖŁĀŹŹĒ mol£Æ(L”¤min)”£
¢ŚČō°“n(SiCl4) : n(N2) : n(H2) =" 3" : 2 : 6µÄĶ¶ĮĻÅä±Č£¬ĻņÉĻŹöČŻĘ÷²»¶ĻĄ©“ó¼ÓĮĻ£¬SiCl4(g)µÄ×Ŗ»ÆĀŹÓ¦ £ØĢī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±£©”£
£Ø6£©298KŹ±£¬Ksp[Ce(OH)4]£½1”Į10”Ŗ29”£Ce(OH)4µÄČܶȻż±ķ“ļŹ½ĪŖKsp£½ ”£
ĪŖĮĖŹ¹ČÜŅŗÖŠCe4£«³ĮµķĶźČ«£¬¼“²ŠĮōŌŚČÜŅŗÖŠµÄc(Ce4+)Š”ÓŚ1”Į10£5mol”¤L£1£¬Ščµ÷½ŚpHĪŖ ŅŌÉĻ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀ±ķŹĒŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£¬Õė¶Ō±ķÖŠµÄ¢Ł”«¢āÖŠŌŖĖŲ£¬ÓĆŌŖĖŲ·ūŗÅ»ņ»ÆѧŹ½»Ų“šŅŌĻĀĪŹĢā£ŗ
| Ö÷×å ÖÜĘŚ | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| ¶ž | | | | | ¢Ł | | ¢Ś | |
| Čż | ¢Ū | ¢Ü | ¢Ż | ¢Ž | | | ¢ß | ¢ą |
| ĖÄ | ¢į | | | | | | ¢ā | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖ£ŗA”¢B”¢C”¢DĖÄÖÖ¶ĢÖÜĘŚŌŖĖŲŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬AÓėDµÄŌ×ÓŠņŹżÖ®ŗĶµČÓŚBÓėCµÄŌ×ÓŠņŹżÖ®ŗĶ£¬ÓÉDŌŖĖŲ×é³ÉµÄµ„ÖŹŌŚĶس£×“æöĻĀ³Ź»ĘĀĢÉ«£¬B”¢C”¢DČżÖÖŌŖĖŲĪ»ÓŚĶ¬Ņ»ÖÜĘŚ£¬B”¢C”¢DČżÖÖŌŖĖŲµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļ·Ö±šĪŖX”¢Y”¢Z£¬X”¢Y”¢ZæÉĮ½Į½Ļą»„·“Ӧɜ³ÉŃĪŗĶĖ®£¬ŹŌĶʶĻ²¢ÓĆĻąÓ¦µÄ»ÆѧÓĆÓļ»Ų“šĻĀĮŠĪŹĢā”£
(1)DŌŖĖŲŌ×ӵĽį¹¹Ź¾ŅāĶ¼ĪŖ______________________£»
(2)XÓėCŌŖĖŲµÄ×īøß¼ŪŃõ»ÆĪļæÉŅŌ·¢Éś·“Ó¦£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ
____________________________________£»
(3)A”¢B”¢CČżÖÖŌŖĖŲµÄŌ×Ó°ė¾¶ÓÉŠ”µ½“óµÄĖ³ŠņĪŖ_________________£»
(4)AÓėDĮ½ŌŖĖŲµÄĘųĢ¬Ēā»ÆĪļÖ®¼äæÉŅŌ·“Ӧɜ³ÉŅ»ÖÖŃĪ£¬øĆŃĪµÄĖ®ČÜŅŗ³Ź________(Ģī”°Ėį”±”°¼ī”±»ņ”°ÖŠ”±)ŠŌ£¬øĆĖ®ČÜŅŗÖŠø÷Ąė×ÓÅضČÓÉŠ”µ½“óµÄĖ³ŠņĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com