
����úΪ��Ҫԭ�ϵĺϳɰ���ҵ�У�ԭ����������������������ã�
��C��H2O(g)![]() CO��H2��
CO��H2��
��CO��H2O(g)![]() CO2��H2��
CO2��H2��
(1)��֪CO(g)��1/2O2(g)��CO2(g)����H����283.0 kJ/mol��
H2(g)��1/2O2(g)��H2O(g)����H����285.8 kJ/mol��д������CO��H2O(g)��Ӧ���Ȼ�ѧ����ʽ��
________________
(2)�ӷ�Ӧ������з����H2�ķ���ͨ�����Լ�Һϴ�������ݸù�ҵ������ʵ�ʷ��������ѡ������������Һ��Ϊ���ռ���________��������________��
A������������Һ
B����ˮ
C��ʯ��ˮ��ʯ����
(3)��ʵ����ģ��������Ӧ�ڣ�830��ʱ��1 L��������װ��CO��H2O(g)��2 molʹ֮��Ӧ���ﵽƽ��ʱ���������CO2��Ũ��Ϊ1 mol/L������830��ʱ�÷�Ӧ��ƽ�ⳣ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| ||
| ||
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�����и߿�ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�������
��ҵ�ϳɰ��ķ�ӦΪ��N2(g)+3H2(g)  2NH3(g) ��H <0
2NH3(g) ��H <0
ijʵ�齫3.0 mol N2(g)��4. 0 mol H2(g)�����ݻ�Ϊ10L���ܱ������У����¶�T1�·�Ӧ�����H2�����ʵ����淴Ӧʱ��ı仯����ͼ��ʾ��
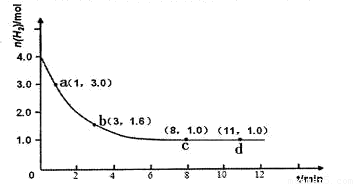
��1����Ӧ��ʼ3min�ڣ�H2��ƽ����Ӧ����Ϊ ��
��2������������ºϳɰ���Ӧ�Ļ�ѧƽ�ⳣ����д��������̣��������2λ��Ч���֣���
��3�����ı��¶�ΪT2 ( T2С��TI���ٽ���ʵ�飬���ڴ����ͼ�л���H2�����ʵ����淴Ӧʱ��仯��Ԥ�ڽ��ʾ��ͼ��
��4������úΪ��Ҫԭ�ϵĺϳɰ���ҵ�У�ԭ����������������������ã�
��C+H2O(g)
 CO+H2����CO+H2O(g)
CO+H2����CO+H2O(g)  CO2+H2��
CO2+H2��
��֪��CO(g)+1/2O2(g)=CO2(g) ��H=��283.0kJ/mol
H2(g)+1/2O2(g)=H2O(g) ��H=��241.8kJ/mol
д������CO��H2O(g)��Ӧ���Ȼ�ѧ����ʽ�� ��
��5���ϳɰ���ҵ�У�ԭ����(N2��H2��������CO��NH3���ڽ���ϳ���֮ǰ���ô��������ͭ��I����Һ������CO���䷴ӦΪ��
CH3COO[Cu(NH3)2]+CO+NH3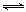 CH3COO[Cu(NH3)3]•CO
��H<0
CH3COO[Cu(NH3)3]•CO
��H<0
д�����CO�����ʵ�����һ���ʩ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�����з�خ������ͳ����һ�����ۻ�ѧ�Ծ��������棩 ���ͣ�������
��ҵ�ϳɰ��ķ�ӦΪ��N2(g)+3H2(g)
 2NH3(g)
��H<0��ijʵ�齫3.0 mol N2(g)��4. 0 mol H2(g)�����ݻ�Ϊ10L���ܱ������У����¶�T1�·�Ӧ�����H2�����ʵ����淴Ӧʱ��ı仯����ͼ��ʾ��
2NH3(g)
��H<0��ijʵ�齫3.0 mol N2(g)��4. 0 mol H2(g)�����ݻ�Ϊ10L���ܱ������У����¶�T1�·�Ӧ�����H2�����ʵ����淴Ӧʱ��ı仯����ͼ��ʾ��
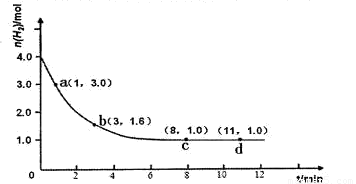
��1����Ӧ��ʼ3min�ڣ�H2��ƽ����Ӧ����Ϊ ��
��2������������ºϳɰ���Ӧ�Ļ�ѧƽ�ⳣ����д��������̣��������2λ��Ч���֣���
��3�����ı��¶�ΪT2 ( T2С��TI���ٽ���ʵ�飬���ڴ����ͼ�л���H2�����ʵ�����
��Ӧʱ��仯��Ԥ�ڽ��ʾ��ͼ��
��4������úΪ��Ҫԭ�ϵĺϳɰ���ҵ�У�ԭ����������������������ã�
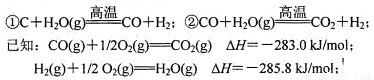
д������CO��H2O(g)��Ӧ���Ȼ�ѧ����ʽ�� ��
��5���ϳɰ���ҵ�У�ԭ����(N2��H2��������CO��NH3���ڽ���ϳ���֮ǰ���ô��������ͭ��I����Һ������CO�䷴ӦΪ��CH3COO[Cu(NH3)2]+CO+NH3 CH3COO[Cu(NH3)3]•CO
��H<0��д�����CO�����ʵ�����һ���ʩ��
��
CH3COO[Cu(NH3)3]•CO
��H<0��д�����CO�����ʵ�����һ���ʩ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ�����з�خ���߿���ѧͳ���Ծ���һ���������棩 ���ͣ������

 CO+H2����CO+H2O��g��
CO+H2����CO+H2O��g�� CO2+H2��
CO2+H2�� O2��g���TCO2��g����H=-283.0KJ/mol��
O2��g���TCO2��g����H=-283.0KJ/mol�� O2��g��=H2O��g����H=-285.8KJ/mol��
O2��g��=H2O��g����H=-285.8KJ/mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ�����и߿���ѧһģ�Ծ��������棩 ���ͣ������

 CO+H2����CO+H2O��g��
CO+H2����CO+H2O��g�� CO2+H2��
CO2+H2�� O2��g���TCO2��g����H=-283.0KJ/mol��
O2��g���TCO2��g����H=-283.0KJ/mol�� O2��g��=H2O��g����H=-285.8KJ/mol��
O2��g��=H2O��g����H=-285.8KJ/mol���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com