
| 16��5 |
| 59.7% |
| 134��35.82% |
| 12 |
| 134��4.48% |
| 1 |
| 44.8L |
| 22.4L/mol |
| 33.6L |
| 22.4L/mol |
| 16��5 |
| 59.7% |
| 134��35.82% |
| 12 |
| 134��4.48% |
| 1 |
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��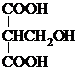 ���֣�
���֣� ����
����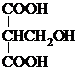 ����
���� ��
�� ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ֽ����NaOH���� |
| B����Һǰ����ƿ����ˮ�� |
| C��ҡ�Ⱥ�Һ����ڿ̶��ߣ��ټ�ˮ���̶��� |
| D������ʱ�����ӿ̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������21���;��й���ǰ���ĺϳɲ��ϣ�
������21���;��й���ǰ���ĺϳɲ��ϣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
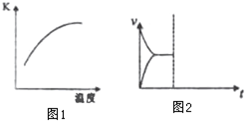 ��ѧ��һֱ�����ڡ��˹��̵������·����о���
��ѧ��һֱ�����ڡ��˹��̵������·����о���| v(N2) |
| v(O2) |
| c(OH-) |
| c(NH3?H2O) |
| c(H+) |
| c(OH-) |
| m |
| n |
| m |
| n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� | H2��g��+O2��g���TH2O��g����H�T-242kJ/mol�� |
| �� | 2H2��g��+O2��g���T2H2O��l����H�T-572kJ/mol�� |
| �� | C��s��+O2��g���TCO��g����H�T-110.5kJ/moL�� |
| �� | C��s��+O2��g���TCO2��g����H�T-393.5kJ/moL�� |
| �� | CO2��g��+2H2O��g���TCH4��g��+2O2��g����H�T+802kJ/moL |
| ��ѧ�� | O=O | C-C | H-H | O-O | C-O | O-H | C-H |
| ����kJ/mol | 497 | 348 | 436 | 142 | 351 | 463 | 414 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com