
��Ƴ������������ƹ��գ����������Ƶķ�ˮʱ�����ڴ���TiO2�����£�����NaClO��CN-����������CNO-��������������CNO-������NaClO������N2��CO2������������Ա���ܱ�ϵͳ������ͼװ�ý���ʵ�飬��֤��������������Ч�ԣ���ͨ���ⶨ������̼����ȷ��CN-�������İٷ��ʡ�

��Ũ����CN-���ӵ���ˮ�����NaClO��Һ�Ļ��Һ��200 mL(����CN-��Ũ��Ϊ0.05 mol•L-1������У�������Ƥ����һ��ʱ�����Ƥ���ͻ�����ʹ��Һȫ���������У��رջ������ش��������⣺
��1�����з�Ӧ�����ӷ���ʽΪ________________________�����з�Ӧ�����ӷ���ʽΪ________________________��
��2���������ɵ������N2��CO2�⣬����HCl��������Cl2�ȡ����м���ij����Լ��DZ���ʳ��ˮ����������_____________________������ʵ���е�������______________��װ�м�ʯ�ҵĸ���ܵ�������______________________________��
��3������ʢ�к�Ca(OH)2 0.02mol��ʯ��ˮ����ʵ�������й�����0.82 g���������ʵ���в��CN-�������İٷ��ʵ���__________���ò��ֵ�빤ҵʵ�ʴ����İٷ����������ƫ�ͣ���Ҫ˵������ԭ��֮һ_______________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ������2016-2017ѧ��߶���ѧ�ڵ�һ���¿���3�£���ѧ�Ծ� ���ͣ�ѡ����
�����³������ؾ����������ṹ�����������Ļ��ϼ۲���Ϊ0�ۣ�����Ϊ��2�ۡ�����ͼ��ʾΪ�������ؾ����һ������(��������С���ظ���Ԫ)��������˵������ȷ����

A���������صĻ�ѧʽΪKO2��ÿ����������4��K����4��O2-
B��������ÿ��K����Χ��8��O2-��ÿ��O��Χ��8��K��
C����������ÿ��K�����������K����8��
D�������У�0�����룭2��������Ŀ��Ϊ2:1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��һ��3�£���ѧ�ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
X��Y��Z��W��Ϊ������Ԫ�أ�������Ԫ�����ڱ��е�λ����ͼ��ʾ����֪Yԭ�ӵ������������Ǵ�����3��������������ȷ����

A. ԭ�Ӱ뾶��W>Z>Y>X
B. ����������Ӧˮ��������ԣ�Z>W>X
C. Y���ʵķе��Z���ʵķе��
D. W���ʸ�ˮ��Ӧ�γɵ���Һ����Ư����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ����3�����������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
��������Ƶ�����֣�����Ӱ�����ǵ�����ͽ�����������Ҫ��Ⱦ��Ϊ�����������PM2.5������Ҫ��ԴΪȼú��������β���ȡ���ˣ���PM2.5��SO2��NOx�Ƚ����о�������Ҫ���塣��ش��������⣺
��1����PM2.5����������ˮ�����Ƴɴ���������
����ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
Ũ��mol/L | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
���ݱ��������ж�������pH=_________��
��2������β����NOx��CO�����ɣ�
����֪����������NO�ķ�ӦΪ��N2(g)+O2(g) 2NO(g) ��H>0���£������ܱ������У�����˵������˵���÷�Ӧ�ﵽ��ѧƽ��״̬����____
2NO(g) ��H>0���£������ܱ������У�����˵������˵���÷�Ӧ�ﵽ��ѧƽ��״̬����____
A.���������ܶȲ��ٱ仯 B.��������ƽ�����������ٱ仯
C.N2��O2��NO�����ʵ���֮��Ϊ1��1��2 D.�����İٷֺ������ٱ仯
������ȼ�Ͳ���ȫȼ��ʱ����CO���������밴���з�Ӧ��ȥCO��2CO(g)=2C(s)+O2(g)����֪�÷�Ӧ�ġ�H��0����������ܷ�ʵ��______________����ܡ����ܡ���
��3��Ϊ����SO2���ŷţ�����ȡ�Ĵ�ʩ�У�
�ٽ�úת��Ϊ�������ȼ�ϡ���֪��
H2(g)+ 1/2O2(g) =H2O(g) ��H=��241.8kJ��mol-1
C(s)+1/2O2(g) =CO(g)��H =-110.5kJ��mol-1
д����̿��ˮ������Ӧ���Ȼ�ѧ����ʽ��___________________��
��ϴ�Ӻ�SO2��������
��4������β����������Ҫԭ����2NO(g)+2CO(g) 2CO2(g)+N2(g)����H��0�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����_________������ţ�������ͼ��v����K��n��w�ֱ��ʾ����Ӧ���ʡ�ƽ�ⳣ�������ʵ���������������
2CO2(g)+N2(g)����H��0�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����_________������ţ�������ͼ��v����K��n��w�ֱ��ʾ����Ӧ���ʡ�ƽ�ⳣ�������ʵ���������������

�����ŷŵĵ������úȼ�ղ����Ķ��������ǵ������������ġ�������ס�֮һ������̿�ɴ���������Ⱦ��NO����5L�ܱ������м���NO�ͻ���̿�����������ʣ���һ����������������E��F�����¶ȷֱ���T1���T2��ʱ����ø�����ƽ��ʱ���ʵ�����n/mol�����±���
���� �¶�/�� | ����̿ | NO | E | F |
��ʼ | 3.000 | 0.10 | 0 | 0 |
T1 | 2.960 | 0.020 | 0.040 | 0.040 |
T2 | 2.975 | 0.050 | 0.025 | 0.025 |
��1��д��NO�����̿��Ӧ�Ļ�ѧ����ʽ______________________��
��2������������ӦT1��ʱ��ƽ�ⳣ��K1=__________________����T1��T2����÷�Ӧ�ġ�H__________________0(���������������������
��3��������ӦT1��ʱ�ﵽ��ѧƽ�����ͨ��0.1molNO���壬��ﵽ�»�ѧƽ��ʱNO��ת����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ����3�����������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ������������ܹ��ﵽ��Ӧʵ��Ŀ�ĵ��ǣ� ����
ʵ������ | ʵ��Ŀ�� | |
A | ��SO2ͨ��Ʒ����Һ�У�Ʒ����ɫ��������ɫ��Ʒ����Һ����Һ�ָ���ɫ | ֤��������IJ��ȶ��� |
B | �����£���Na2CO3��Һ�м�����BaSO4��ĩ�����ˣ���ϴ���ij����м�ϡ���ᣬ���������ݲ��� | ֤�������� KSP(BaSO4)��KSP(BaCO3) |
C | �����²ⶨ���ʵ���Ũ����ͬ������ʹ�����Һ��pH������pHС�ڴ���pH | ֤����ͬ�����£���ˮ��HCl����̶ȴ���CH3COOH |
D | ��Ũ�����̼���ʻ�ϼ��ȣ�ֱ�ӽ����ɵ�����ͨ�������ij���ʯ��ˮ��ʯ��ˮ����� | �������������CO2�Ĵ��� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������ʡ��ɽ�и���3���¿������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����и��л���ͬ���칹����Ŀ�������������칹�����ж�����ȷ����
A. ����ʽΪC4H8���ܷ����ӳɷ�Ӧ��ͬ���칹����2��
B. ����ʽΪC8H10�Ķ��ױ���������.��һ����ԭ�ӱ���ԭ��ȡ�������ò�����6��
C. ����ʽΪC4H8Cl2��ֻ��һ������ͬ���칹����5��
D. ����ʽΪC5H10O2��ֻ��һ�������ţ���������ˮ��ͬ���칹����2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����������Ԫ��A��B��C��D��E��ԭ������������������A��Cͬ���壬A������Ԫ�ز���ͬһ���ڣ�B��Dͬ���壬������D�ĵ���Ϊ����ɫ���壮�����ƶ�����ȷ���ǣ� ��
A. ԭ�Ӱ뾶��С�����˳��r��C����r��D����r��E��
B. Ԫ��D��E�ֱ���A�γɵĻ���������ȶ��ԣ�E��D
C. Ԫ��D������������Ӧˮ��������Ա�E��ǿ
D. Ԫ��B�ֱ���A��C�γɵĻ������л�ѧ����������ȫ��ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ʵĵ���ʽ��д��ȷ���ǣ� ��
A.  B.
B.  C.
C. 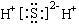 D.
D. 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�����и�һ3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���ֶ�����Ԫ�ص�ijЩ���������ʾ������ֻ��W��Y��ZΪͬ����Ԫ�أ�����˵����ȷ����( )

A. ��Q��Y�γɵĻ�������ֻ�������Ӽ� B. Z��X֮���γɵĻ�������л�ԭ��
C. ��X��Y��Z����Ԫ���γɵĻ����һ���ǹ��ۻ����� D. Y��W�γɵĻ������У�Y�Ը���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com