
��ȡ������(a g)�������ݣ���һ������b gˮ�У���֪�ܽ�ʱ����Q1 KJ����������һ�ݵ���������ȥ�ᾧˮ����ȴ������b gˮ�У��ܽ�ʱ�ͷ�Q2 KJ������
(1)�����ܽ���Ϊ________KJ/mol����________���̣�
(2)��ˮ����ͭ�ܽ���Ϊ________KJ/mol����________���̣�
(3)���������ݿ�֪��CuSO4��5H2O��ϳ�CuSO4��5H2O��ЧӦΪ________KJ/mol���ù��̷��Ȼ������Ⱦ�����________��
 �ο�������ϵ�д�
�ο�������ϵ�д� ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
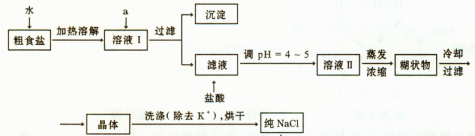
��1����ʳ�γ���������K+��Ca2+��Mg2+��Fe3+��SO2-4'���������ӣ�ʵ�����ᴿNaCl���������£�
�ṩ���Լ�������Na2CO3��Һ ����K2CO3��Һ NaOH��Һ BaCl2��Һ Ba��NO3��2 ��Һ 75%�Ҵ� ���Ȼ�̼
������ȥ��ҺI�е�Ca2+��Mg2+��Fe3+��SO2-4���ӣ�ѡ��a���������Լ������μ�˳������Ϊ ���ѧʽ������Һ�еμ������pH=4 ~5��Ŀ���� ��
��ѡ��75%�Ҵ�ϴ�ӳ�ȥNaCl������渽��������KCl������NaCl�Ƿ�ϴ���IJ����� ��
��2�����ᴿ��NaCl����480mL 4.00mol��L-1NaCl��Һ����Ҫ��ȡ������Ϊ g������������ҩ�ס����������ձ���� �����������ƣ���
��3����ⱥ��ʳ��ˮ��װ����ͼ��ʾ�����ռ���H2Ϊ2L����ͬ���������ռ���Cl2____���>������=����<����2L����Ҫԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��14�֣�ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
��1����ʳ�γ���������K+��Ca2+��Mg2+��Fe3+��SO2-4'���������ӣ�ʵ�����ᴿNaCl���� �����£�
�����£�
�ṩ���Լ�������Na2CO3��Һ ����K2CO3��Һ NaOH��Һ BaCl2��Һ Ba��NO3��2��Һ 75%�Ҵ� ���Ȼ�̼
������ȥ��ҺI�е�Ca2+��Mg2+��Fe3+��SO2-4���ӣ�ѡ��a���������Լ������μ�˳������Ϊ ���ѧʽ������Һ�еμ������pH ="4" ~5��Ŀ���� ��
��ѡ��75%�Ҵ�ϴ�ӳ�ȥNaCl������渽��������KCl������NaCl�Ƿ�ϴ���IJ����� ��
��2�����ᴿ��NaCl����480mL 4.00mol��L-1NaCl��Һ����Ҫ��ȡ������Ϊ g������������ҩ�ס����������ձ���� ������ �����ƣ���
�����ƣ���
��3����ⱥ��ʳ��ˮ��װ����ͼ��ʾ�����ռ���H2Ϊ2L����ͬ���������ռ���Cl2____���>������=����<����2L����Ҫԭ���� ��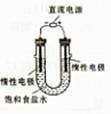
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��14�֣�ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
��1����ʳ�γ���������K+��Ca2+��Mg2+��Fe3+��SO2-4'���������ӣ�ʵ�����ᴿNaCl���������£�
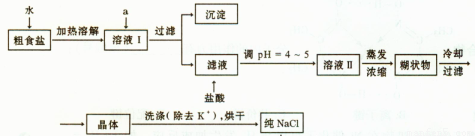
�ṩ���Լ�������Na2CO3��Һ ����K2CO3��Һ NaOH��Һ BaCl2��Һ Ba��NO3��2 ��Һ 75%�Ҵ� ���Ȼ�̼
������ȥ��ҺI�е�Ca2+��Mg2+��Fe3+��SO2-4���ӣ�ѡ��a���������Լ������μ�˳������Ϊ ���ѧʽ������Һ�еμ������pH =4 ~5��Ŀ���� ��
��ѡ��75%�Ҵ�ϴ�ӳ�ȥNaCl������渽��������KCl������NaCl�Ƿ�ϴ���IJ����� ��
��2�����ᴿ��NaCl����480mL 4.00mol��L-1NaCl��Һ����Ҫ��ȡ������Ϊ g������������ҩ�ס����������ձ���� �����������ƣ���
��3����ⱥ��ʳ��ˮ��װ����ͼ��ʾ�����ռ���H2Ϊ2L����ͬ���������ռ���Cl2____���>������=����<����2L����Ҫԭ���� ��
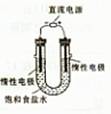
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com