
��֪����ȩ��һ�������¿ɱ���������Ϊ���ᡣA��һ����Ҫ�Ļ���ԭ�ϣ�A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��E�Ǿ��й�����ζ�����������A��B��C��D��һ�������´�������ת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ���
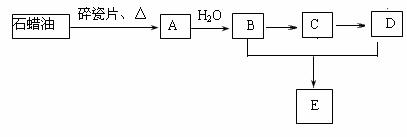
��A��B�ķ�Ӧ������_______________��B��D�й����ŵ����Ʒֱ���_______��________��
��д����ӦB��D��E�Ļ�ѧ����ʽ��_______________________________________��
�Ƕ�������ʯ���ͻ��A�Ĺ����е��м����֮һ������һ��ͬ���칹���к�����������������ͬ���칹��Ľṹ��ʽ��____________________________________________��
���������������˶�����һ��ʱ���Ⱥ첲�е������ͻ���ʹ�ĸо���ԭ��֮һ��
![]() ����֪��������к�����B��D����ͬ�Ĺ����ţ�����һ������������Ľṹ��ʽ�� ��90 g������������������ȫ��Ӧ�����������ڱ�״���µ������ L��
����֪��������к�����B��D����ͬ�Ĺ����ţ�����һ������������Ľṹ��ʽ�� ��90 g������������������ȫ��Ӧ�����������ڱ�״���µ������ L��
 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Cu |
| �� |
| Cu |
| �� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| Cu |
| �� |
| Cu |
| �� |
| ϡ���� |
| �� |
| ϡ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
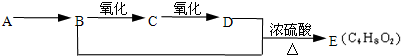
��9�֣���֪����ȩ��һ�������¿ɱ���������Ϊ���ᡣA��ʯ���ѽ����Ҫ����֮һ������������ں���һ������ʯ�ͻ�����չˮƽ�ı�־���������л���֮���ת����ϵ��
��1��A�Ľṹ��ʽΪ ��������Ϊˮ���� ��
��2��B+D��E�ķ�Ӧ����Ϊ ��
��3��д��B��C��B+D��E��������ѧ��Ӧ����ʽ��
B��C�� ��
B+D��E�� ��
��4����ȥE��������������D���ʣ������Լ��������ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�����и�һ��ѧ����ĩ���Ի�ѧ���� ���ͣ������
��9�֣���֪����ȩ��һ�������¿ɱ���������Ϊ���ᡣA��ʯ���ѽ����Ҫ����֮һ������������ں���һ������ʯ�ͻ�����չˮƽ�ı�־���������л���֮���ת����ϵ��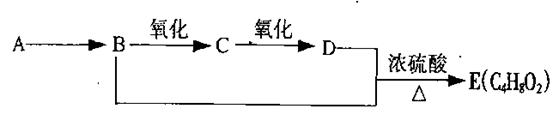
��1��A�Ľṹ��ʽΪ ��������Ϊˮ���� ��
��2��B+D��E�ķ�Ӧ����Ϊ ��
��3��д��B��C��B+D��E��������ѧ��Ӧ����ʽ��
B��C�� ��
B+D��E�� ��
��4����ȥE��������������D���ʣ������Լ��������ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ӱ�ʡ��һ��ѧ����ĩ���Ի�ѧ���� ���ͣ������
��10�֣���֪����ȩ��һ�������¿ɱ���������Ϊ���ᡣA��ʯ���ѽ���Ҫ����֮һ������������ں���һ������ʯ�ͻ�����չˮƽ�ı�־���������л���֮���ת����ϵ��

��1��A�Ľṹ��ʽΪ ��������Ϊˮ���� ��
��2��B+D��E�ķ�Ӧ����Ϊ ��
��3��д��B��C��B+D��E��������ѧ��Ӧ����ʽ��
B��C�� ��
B+D��E�� ��
��4����γ�ȥE��������������D���ʣ������������̣� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com