��ѧ��ѧ�г����ļ������ʴ������¹�ϵ�����м��Ǻ�ɫ�ǽ������ʣ����������г����Ľ������ʣ�D�Ǻ���ɫ���壮��ͼ�в��ֲ���ͷ�Ӧ��������ȥ��

�ش��������⣺
��1��д������A��Ũ��Һ��Ӧ�Ļ�ѧ����ʽ
C+4HNO
3��Ũ��
CO
2��+4NO
2��+2H
2O
C+4HNO
3��Ũ��
CO
2��+4NO
2��+2H
2O
��
��2�����������Ũ�ȵ�G��Һ��H��Һ��Ϻ�õ�����Һ�е�����Ũ�ȴ�С��ϵΪ
c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+��
c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+��
��
��3����ȥG�����к���H���ʲ��õķ�����
����
����
��
��4��A��Һ��һ����ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����巴Ӧ������һ���Σ����ε���Һ�����ԣ���ԭ���ǣ������ӷ���ʽ��ʾ��
NH
4++H
2O

NH
3?H
2O+H
+NH
4++H
2O

NH
3?H
2O+H
+��
��5��д����E��Һ�м�������ϡ�����Ӧ�����ӷ���ʽ
3Fe2++NO3-+4H+=3Fe3++NO��+2H2O
3Fe2++NO3-+4H+=3Fe3++NO��+2H2O
������ҺF�������ɡ����յ��������ټ���ʱ���ù������ʵĻ�ѧʽΪ
Fe2O3
Fe2O3
��
��6��ȷ��E��������ʵ��ķ���Ϊ
�ȼ���KSCN��Һ�ޱ仯�ټ�����ˮ��������ȣ���Һ���ɫ
�ȼ���KSCN��Һ�ޱ仯�ټ�����ˮ��������ȣ���Һ���ɫ
��


 NH3?H2O+H+
NH3?H2O+H+ NH3?H2O+H+
NH3?H2O+H+ NH3?H2O+H+��
NH3?H2O+H+��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
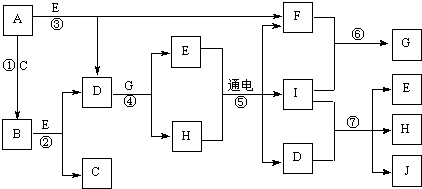


 A-K����ѧ��ѧ�г����ļ������ʣ�����֮���ת����ϵ��ͼ��ʾ����֪������AΪ���嵥�ʣ�BΪ����ɫ��ĩ��G��HΪ��̬���ʣ�I�ڳ�����ΪҺ�壬D��E��F��ˮ��Һ���ʼ��ԣ���C�ı�����Һ��ȡF��E����Ҫ�Ļ����������ش��������⣺
A-K����ѧ��ѧ�г����ļ������ʣ�����֮���ת����ϵ��ͼ��ʾ����֪������AΪ���嵥�ʣ�BΪ����ɫ��ĩ��G��HΪ��̬���ʣ�I�ڳ�����ΪҺ�壬D��E��F��ˮ��Һ���ʼ��ԣ���C�ı�����Һ��ȡF��E����Ҫ�Ļ����������ش��������⣺






