
(8��)��ƹ㷺��Ӧ���ڹ�ҵ�����С��ڵ�Ƹ��Ĺ�ҵ��ˮ����Ҫ����Cr3+,ͬʱ������������Cu2+��Fe2+ ��Fe3+��Al3+�ȣ������Խ�ǿ��Ϊ�������ø�Ԫ�����˹�ҵ��ˮͨ�������������̴�����

��֪��
(1) ��Ԫ��������ѭ�����̣�

(2) Cu(OH)2�����ڰ�ˮ��

��ش��������⣺
(1)�����Լ���Ŀ�ģ�________________
(2)�Լ��ҵ����ƣ�__________ ,�Լ����Ļ�ѧʽ��________________
(3) ��������I��II��III��ͬ������и�ʵ���������Ҫ����Ҫ����������___________
(4)����I����Ҫ�ɷ�Ϊ________________(д��ѧʽ)��
(5)�ڸ�Ԫ��ѭ�����̵ķ�Ӧ����ÿ��Ӧ��1 mol H2O2ͬʱ����H+��ĿΪ��________________
(6) ��Ԫ��ѭ�����̵ķ�Ӧ�����ӷ���ʽ��________________����ˮ������������������III�����ӷ���ʽ��________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)��ƹ㷺��Ӧ���ڹ�ҵ�����С��ڵ�Ƹ��Ĺ�ҵ��ˮ����Ҫ����Cr3+,ͬʱ������������Cu2+��Fe2+ ��Fe3+��Al3+�ȣ������Խ�ǿ��Ϊ�������ø�Ԫ�����˹�ҵ��ˮͨ�������������̴�����
��֪��
(1) ��Ԫ��������ѭ�����̣�
(2) Cu(OH)2�����ڰ�ˮ��
��ش��������⣺
(1)�����Լ���Ŀ�ģ�________________
(2)�Լ��ҵ����ƣ�__________ ,�Լ����Ļ�ѧʽ��________________
(3) ��������I��II��III��ͬ������и�ʵ���������Ҫ����Ҫ����������___________
(4)����I����Ҫ�ɷ�Ϊ________________(д��ѧʽ)��
(5)�ڸ�Ԫ��ѭ�����̵ķ�Ӧ����ÿ��Ӧ��1 mol H2O2ͬʱ����H+��ĿΪ��________________
(6) ��Ԫ��ѭ�����̵ķ�Ӧ�����ӷ���ʽ��________________����ˮ������������������III�����ӷ���ʽ��________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ������ѧ����У������һ��������ѧ�Ծ����������� ���ͣ������
����10�֣��״��ϳɷ�Ӧ���������仯��ͼ��ʾ��
��1��д���ϳɼ״����Ȼ�ѧ����ʽ________________________________��
ʵ������1 L���ܱ������н���ģ��ϳ�ʵ�顣��1 mol CO��2 mol H2ͨ�������У��ֱ������300 ���500 �淴Ӧ��ÿ��һ��ʱ����������CH3OH��Ũ�����±���ʾ��
| ʱ��Ũ��(mol/L)�¶� | 10 min | 20 min | 30 min | 40 min | 50 min | 60 min |
| 300 �� | 0.40 | 0.60 | 0.75 | 0.84 | 0.90 | 0.90 |
| 500 �� | 0.60 | 0.75 | 0.78 | 0.80 | 0.80 | 0.80 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���½����Ŷ��и�����һ���¿���ѧ�Ծ� ���ͣ������
(8��)��ƹ㷺��Ӧ���ڹ�ҵ�����С��ڵ�Ƹ��Ĺ�ҵ��ˮ����Ҫ����Cr3+,ͬʱ������������Cu2+��Fe2+��Fe3+��Al3+�ȣ������Խ�ǿ��Ϊ�������ø�Ԫ�����˹�ҵ��ˮͨ�������������̴�����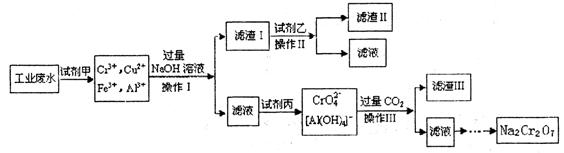
��֪��
(1) ��Ԫ��������ѭ�����̣�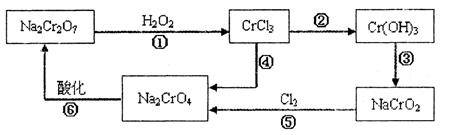
(2) Cu(OH)2�����ڰ�ˮ��
��ش��������⣺
(1)�����Լ���Ŀ�ģ�________________
(2)�Լ��ҵ����ƣ�__________ ,�Լ����Ļ�ѧʽ��________________
(3) ��������I��II��III��ͬ������и�ʵ���������Ҫ����Ҫ����������___________
(4)����I����Ҫ�ɷ�Ϊ________________(д��ѧʽ)��
(5)�ڸ�Ԫ��ѭ�����̵ķ�Ӧ����ÿ��Ӧ��1 mol H2O2ͬʱ����H+��ĿΪ��________________
(6) ��Ԫ��ѭ�����̵ķ�Ӧ�����ӷ���ʽ��________________����ˮ������������������III�����ӷ���ʽ��________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ����У������һ��������ѧ�Ծ��������棩 ���ͣ������
����10�֣��״��ϳɷ�Ӧ���������仯��ͼ��ʾ��

��1��д���ϳɼ״����Ȼ�ѧ����ʽ________________________________��
ʵ������1 L���ܱ������н���ģ��ϳ�ʵ�顣��1 mol CO��2 mol H2ͨ�������У��ֱ������300 ���500 �淴Ӧ��ÿ��һ��ʱ����������CH3OH��Ũ�����±���ʾ��
|
ʱ��Ũ��(mol/L)�¶� |
10 min |
20 min |
30 min |
40 min |
50 min |
60 min |
|
300 �� |
0.40 |
0.60 |
0.75 |
0.84 |
0.90 |
0.90 |
|
500 �� |
0.60 |
0.75 |
0.78 |
0.80 |
0.80 |
0.80 |
��2����300 �淴Ӧ��ʼ10 min�ڣ�H2��ƽ����Ӧ����Ϊv(H2)��________��
��3����500 ��ﵽƽ��ʱ��ƽ�ⳣ��K��________��
��4������һ���������ܱ������У�����1.2 mol CO��2.0 mol H2��һ�������´ﵽƽ�⣬���������ѹǿΪ��ʼѹǿ��һ�롣�����������H2��ת����Ϊ________��
��5��ͭ���������л��Ըߡ�ѡ���Ժú������º͵��ص㣬�ѹ㷺��ʹ����CO/CO2�ļ���ϳɼ״����÷�Ӧ��a�Ĵ�С�Է�Ӧ�Ȧ�H����Ӱ�죬___________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com