
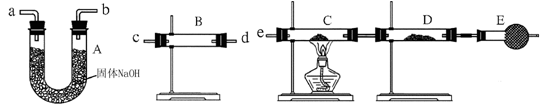
| װ�� | ��ʢҩƷ | ʵ������ | ���� |
| B | | | |
| C | CuO���� | | |
| D | ��ˮCuSO4 | |
| װ�� | ��ʢҩƷ | ʵ������ | ���� |
| B | ��ˮCuSO4 | ��ɫ��ĩ�����ɫ | �����к���ˮ���� |
| C | | ��ɫ�����ɺ�ɫ | �����к������� |
| D | | ��ɫ��ĩ�����ɫ |
| װ�� | ��ʢҩƷ | ʵ������ | ���� |
| B | ��ˮCuSO4 | ��ɫ��ĩ�����ɫ | �����к���ˮ���� |
| C | | ��ɫ�����ɺ�ɫ | �����к������� |
| D | | ��ɫ��ĩ�����ɫ |


 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
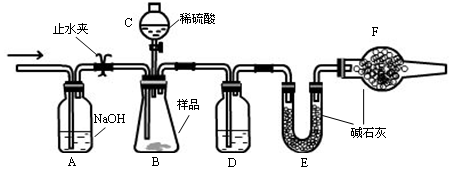
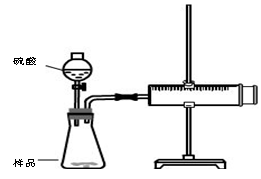
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 �ⶨ������������
�ⶨ������������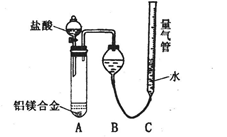
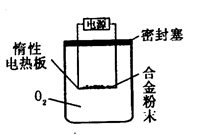
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �����ϡ� | ��ˮ���嵥�ʵ�ˮ��Һ���������嵥�ʶ��ʻ�ɫ�� |
| �Լ� | ��̪��Һ��CCl4����ˮ�ƾ���KSCN��Һ | ||
| ���� | �������� | ʵ������ | ���� |
| 1 | ȡ������ɫ��Һ���Թ��У��μ� ���� | ��Һ���ɫ | ��Ӧ ���� |
| 2 | ȡ������ɫ��Һ���Թ��У��μ� ���� | ________ ________ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
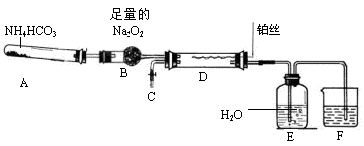
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
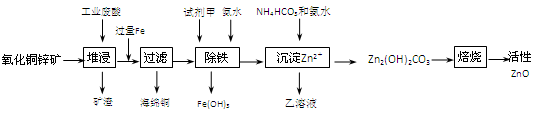
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2�� | 6��34 | 9��7 |
| Fe3�� | 1��48 | 3��2 |
| Zn2�� | 6��2 | 8��0 |
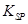 = 2��70��10-39]
= 2��70��10-39]�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A��FeCl3 | B��FeCl2 | C��CuCl2 | D��FeS |
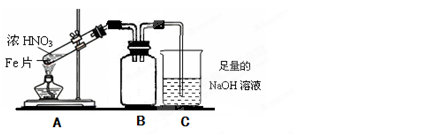
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
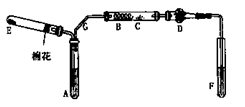
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ������Ա���ǿ�����ܷ�Ӧ���ɰ�ɫBaSO3����������ͼ��ʾװ�ý���ʵ�飨�г�װ�ú�A�м���װ�����ԣ��������Ѽ��飩
������Ա���ǿ�����ܷ�Ӧ���ɰ�ɫBaSO3����������ͼ��ʾװ�ý���ʵ�飨�г�װ�ú�A�м���װ�����ԣ��������Ѽ��飩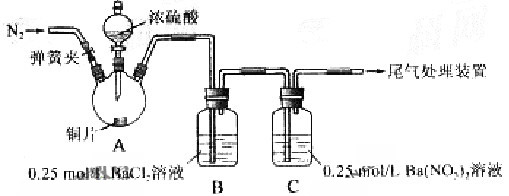
| ���� | ���� |
| �رյ��ɼУ��μ�һ����Ũ���ᣬ���� | A���а������ɣ�ͭƬ����������� B��������ð��������������ɫ���� C��  ������ɫ������Һ���Ϸ�����dz��ɫ������ʧ ������ɫ������Һ���Ϸ�����dz��ɫ������ʧ |
| ���ɼУ�ͨ��N2, ֹͣ���ȣ�һ��ʱ���ر� | ___________________ |
| ��B��C�зֱ�ȡ������ɫ��������ϡ���� | ��δ���ְ�ɫ�����ܽ� |
| �� | ������ɫ���� |
| �� | ������ɫ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com