

 ��-CH�TCH2
��-CH�TCH2
 +NaOH
+NaOH| �� |
| �� |
 +NaCl+H2O
+NaCl+H2O +NaOH
+NaOH| �� |
| �� |
 +NaCl+H2O
+NaCl+H2O ��
��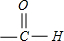 ��Ϊȩ����ȩ���������������ӳɷ�����ԭ��Ӧ��Ҳ�ɱ�����Ϊ���ᣬ
��Ϊȩ����ȩ���������������ӳɷ�����ԭ��Ӧ��Ҳ�ɱ�����Ϊ���ᣬ �����ӽṹΪ
�����ӽṹΪ ������ƽ��ṹ��HCHOҲ��ƽ��ṹ������
������ƽ��ṹ��HCHOҲ��ƽ��ṹ������ ����Ϊƽ��ṹ���ʢ���ȷ��
����Ϊƽ��ṹ���ʢ���ȷ�� ���������ƴ���Һ�����£�������ȥ��Ӧ���ɻ���ϩ����Ӧ����ʽΪ
���������ƴ���Һ�����£�������ȥ��Ӧ���ɻ���ϩ����Ӧ����ʽΪ +NaOH
+NaOH| �� |
| �� |
 +NaCl+H2O��
+NaCl+H2O�� +NaOH
+NaOH| �� |
| �� |
 +NaCl+H2O��
+NaCl+H2O��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 ��
��





�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣���֪��

��1���÷�Ӧ���������к��еĹ����ŵ�������__________����������һ���������ܷ���_______������ţ���
��������Ӧ �ڻ�ԭ��Ӧ ��������Ӧ
��2����֪HCHO����������ԭ�Ӷ���ͬһƽ���ڣ���ҪʹR1CHO����������ԭ�ӿ��ܶ���ͬһƽ���ڣ�R1������________������ţ���
![]() �١�CH3 �� �ۡ�CH�TCH2
�١�CH3 �� �ۡ�CH�TCH2
��3��ij�ȴ���A�ķ���ʽΪC6H11Cl�������Է�������ת����
�ṹ��������E�����к�����������û��֧����
��д��A![]() B�Ļ�ѧ����ʽ��
B�Ļ�ѧ����ʽ��
��E�Ľṹ��ʽΪ��
��д��D��ͬʱ��������������ͬ���칹��Ľṹ��ʽ�� a.���ڶ�Ԫ���� b.��������4��̼ԭ�ӣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ����һ�и�����ѧ�ڵ�����ģ�⿼�Ի�ѧ�Ծ� ���ͣ������
��8�֣���֪��
��1���÷�Ӧ���������к��еĹ����ŵ�������__________����������һ���������ܷ���_______������ţ���
��������Ӧ �ڻ�ԭ��Ӧ ��������Ӧ
��2����֪HCHO����������ԭ�Ӷ���ͬһƽ���ڣ���ҪʹR1CHO����������ԭ�ӿ��ܶ���ͬһƽ���ڣ�R1������________�� ����ţ���
����ţ��� �١�CH3 �� �ۡ�CH�TCH2
�١�CH3 �� �ۡ�CH�TCH2
��3��ij�ȴ���A�ķ���ʽΪC6H11Cl�������Է�������ת����
 |
 B�Ļ�ѧ����ʽ��
B�Ļ�ѧ����ʽ�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�ڵ�����ģ�⿼�Ի�ѧ�Ծ� ���ͣ������
��8�֣���֪��

��1���÷�Ӧ���������к��еĹ����ŵ�������__________����������һ���������ܷ���_______������ţ���
��������Ӧ �ڻ�ԭ��Ӧ ��������Ӧ
��2����֪HCHO����������ԭ�Ӷ���ͬһƽ���ڣ���ҪʹR1CHO����������ԭ�ӿ��ܶ���ͬһƽ���ڣ�R1������________������ţ���
 �١�CH3 �� �ۡ�CH�TCH2
�١�CH3 �� �ۡ�CH�TCH2
��3��ij�ȴ���A�ķ���ʽΪC6H11Cl�������Է�������ת����
 |
�ṹ��������E�����к�����������û��֧����
��д��A B�Ļ�ѧ����ʽ��
B�Ļ�ѧ����ʽ��
��E�Ľṹ��ʽΪ��
��д��D��ͬʱ��������������ͬ���칹��Ľṹ��ʽ�� a.���ڶ�Ԫ���� b.��������4��̼ԭ�ӣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com