
���� ���ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ�����ͬ�ǽ���Ԫ��֮�����γɼ��Լ���ͬ�ַǽ���Ԫ��֮�����γɷǼ��Լ����������Ӽ��ľ��������Ӿ��壬ֻ�����ۼ��Ļ������ǹ��ۻ����������������غϵķ���Ϊ�Ǽ��Է��ӣ�����������IJ��غϵķ���Ϊ���Է��ӣ��ݴ˷������
��� �⣺NaCl��ֻ�����Ӽ���Ϊ���Ӿ��壻
NaOH�к������Ӽ��ͼ��Լ���Ϊ���Ӿ��壻
Na2O2�к������Ӽ��ͷǼ��Լ���Ϊ���Ӿ��壻
H2O2�к��м��Լ��ͷǼ��Լ���Ϊ�Ǽ��Է��ӣ�
��NH4��2S�к������Ӽ������Լ�����λ����Ϊ���Ӿ��壻
CCl4��ֻ�����Լ���Ϊ�Ǽ��Լ����ӣ�
C2H2�к��м��Լ��ͷǼ��Լ���Ϊ�Ǽ��Է��ӣ�
SiC���м��Լ���Ϊԭ�Ӿ��壻
����衢���ʯ�ж�ֻ���Ǽ��Լ���Ϊԭ�Ӿ��壻
SO3�к��м��Լ���Ϊ�Ǽ��Է��ӣ�
������ֻ�������Ӽ������Ӿ�����NaCl���ʴ�Ϊ��NaCl��
�����м������Ӽ������м��Լ�����λ�������Ӿ����ǣ�NH4��2S��
�ʴ�Ϊ����NH4��2S��
�����м������Ӽ������зǼ��Լ�����Na2O2���ʴ�Ϊ��Na2O2��
�����к��зǼ��Լ��ķǼ��Է��ӵ���C2H2���ʴ�Ϊ��C2H2��
�����к��Ǽ��Լ��ļ��Է��ӵ���H2O2���ʴ�Ϊ��H2O2��
�����к��м��Լ���ԭ�Ӿ�����SiC���ʴ�Ϊ��SiC��
���� ���⿼�黯ѧ�������������жϣ�Ϊ��Ƶ���㣬���ؿ�����������ȷ���Ӽ����ۼ����𡢾������͵��жϷ������ɽ��ע�⣺˫��ˮΪ���Է��ӣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ձ����ƾ��ơ��Թܡ�����̨����Ȧ����©�� | |
| B�� | �ձ���©����������������̨����Ȧ������ֽ | |
| C�� | ��ֽ���ձ����ԹܼС�©���������� | |
| D�� | ��ֽ���Թܡ�©��������̨����Ȧ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
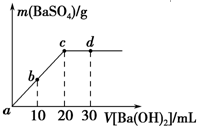 �����£���0.25mol•L-1��������Һ����μ������ʵ���Ũ����ͬ������������Һ�����ɳ����������������������Һ�������ϵ��ͼ��ʾ��a��b��c��d�ֱ��ʾʵ�鲻ͬʱ�̵���Һ�������й�˵���в���ȷ���ǣ�������
�����£���0.25mol•L-1��������Һ����μ������ʵ���Ũ����ͬ������������Һ�����ɳ����������������������Һ�������ϵ��ͼ��ʾ��a��b��c��d�ֱ��ʾʵ�鲻ͬʱ�̵���Һ�������й�˵���в���ȷ���ǣ�������| A�� | ������Һ�����Ϊ20mL | |
| B�� | bʱ����Һ��SO${\;}_{4}^{2-}$��Ũ��ԼΪ0.083mol•L-1 | |
| C�� | dʱ����Һ��pHΪ12 | |
| D�� | ��Ӧ�����ӷ�Ӧ����ʽΪBa2++SO4-+2H++2OH-�TBaSO4��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K+��Cu2+��Na+��Cl- | B�� | H+��Na+��NO3-��CO32- | ||
| C�� | Ba2+��H+��NO3-��SO42- | D�� | Mg2+��Na+��OH-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 50mL 1.0mol/L��HCl | B�� | 10mL 2.0mol/L ��NaCl | ||
| C�� | 20mL 1.5mol/L ��HNO3 | D�� | 10mL 1.0mol/L��H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 11.2L��22.4L | B�� | 15.68L��17.92L | C�� | 13.44L��20.16L | D�� | 16.8L��16.8L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| t���棩 | 0 | 10 | 20 | 25 | 40 | 50 | 90 | 100 |
| Kw/10-14 | 0.134 | 0.292 | 0.681 | 1.01 | 2.92 | 5.47 | 38.0 | 55.0 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com