
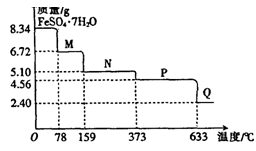
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������һ����Cu |
| B����������һ������ͭ������һ�������� |
| C��������һ����Fe |
| D����Һ��һ������Fe2+������һ������Cu2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�ڿ����а���������5000C���γ�Fe3O4���¶������ߣ����γ�Fe2O3���ڸ��ߵ��¶��¼���Fe2O3��Լ14000C��ʱ����ʧȥ�����ֵõ�Fe3O4������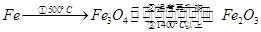 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1:4 | B��2:7 | C��1:2 | D��3:8 |
�鿴�𰸺ͽ���>>
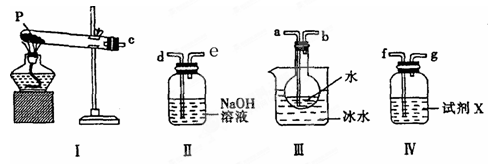
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
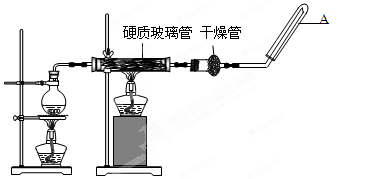
 ��
�� ��ָ����������ԭ��Ӧ�Ļ�ԭ���� ���������� ��
��ָ����������ԭ��Ӧ�Ļ�ԭ���� ���������� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
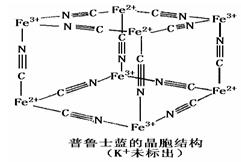
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���٢ڢ� | C���٢ۢ� | D���٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ��֪ | ���� |
| A | ��Fe�� �뵽CuSO4��Һ�� �뵽CuSO4��Һ��Fe + Cu2+ =" Cu" + Fe2+ | ��Na���뵽CuSO4��Һ�� 2Na + Cu2+ =" Cu" + 2Na+ |
| B | ����ȼ�ϵ�صĸ�����Ӧ��ϡ�������������Һ����H2 �C 2e�� = 2H+ | ����ȼ�ϵ�صĸ�����Ӧ������������Һ���������Һ����H2 �C 2e�� + 2OH��=2H2O |
| C | �ö��Ե缫���CuSO4��Һ 2Cu2++ 2H2O  4H+ + O2��+ 2Cu 4H+ + O2��+ 2Cu | �ö��Ե缫���CuCl2��Һ 2Cu2+ + 2H2O  4H+ + O2��+ 2Cu 4H+ + O2��+ 2Cu |
| D | ������CaCO3��ĩͶ������������ CaCO3+ 2H+ = Ca2+ + H2O + CO2�� | ������CaCO3��ĩͶ������������Һ�� CaCO3 + 2H+ = Ca2+ + H2O + CO2�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com