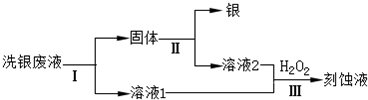
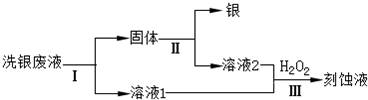
�⣺��1��FeCl
3��Һ����������Ϊ������ˮ���������������������ӣ���Ӧ�����ӷ���ʽΪ��Fe
3++3H
2O?Fe��OH��
3+3H
+��
�ʴ�Ϊ��Fe
3++3H
2O?Fe��OH��
3+3H
+��
��2��FeCl
3��Һϴ����������ӦΪ��Fe
3++Ag?Fe
2++Ag
+��
a��c��Fe
3+����С����a���ϣ�������
b�� c��Cl
-�����䣬��b�����ϣ�������
c����Ԫ�ش�����ʽ��ͬ�����������С����c�����ϣ�
�ʴ�Ϊ��a��
��3��ͨ������Fe��NO
3��
3��Һϴ����NO
3-�Ļ�ԭ�������ж�NO
3-�Ƿ��ܽ�����������Ҫ������Һ�У���������Ҳ�ᷴӦ��
�ʴ�Ϊ������������NO
3-Ҳ������Fe
2+����������ԭ��Ӧ��
��4����ϴ����Һ��Fe
3+��Fe
2+��Ag
+��NO
3-���л������Ϳ�ʴҺ��Ҫ�ȼ����������ԭFe
3+��Ag
+����Ӧ�����ӷ���ʽΪ��2Fe
3++Fe=3Fe
2+Fe+2Ag
+=Fe
2++2Ag��
�ʴ�Ϊ��2Fe
3++Fe=3Fe
2+Fe+2Ag
+=Fe
2++2Ag��
�ڹ��̢��м�����Լ����ܽ����������������ϡ�����ϡ���
�ʴ�Ϊ��ϡ�����ϡ���
��5����������Һ�м���������������Ȼ�����ͬʱ�����Ȼ�狀�ˮ����Ӧ�Ļ�ѧ����ʽΪ��Ag��NH
3��
2OH+3HCl=AgCl��+2NH
4Cl+H
2O��
�ʴ�Ϊ��Ag��NH
3��
2OH+3HCl=AgCl��+2NH
4Cl+H
2O��
�������AgCl�м����ǰ���NH
2OH������ַ�Ӧ��ɵ������ǰ�������ΪN
2����ӦΪ��2AgCl+2NH
2OH=N
2��+2Ag+2H
2O+2HCl����Ӧ������3.3g�ǰ����ʵ���=
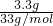
=0.1mol�����������ʵ���Ϊ0.1mol������Ϊ10.8g��
�ʴ�Ϊ��10.8��
��������1��������ˮ���������������������ӣ���Һ�����ԣ�
��2�����ݷ�ӦFe
3++Ag?Fe
2++Ag
+�������ж������Ӳ��䣬��Ԫ���������䣻
��3�����������������Һ�о��������ԣ��������������������ӣ�
��4����ϴ����Һ��Fe
3+��Fe
2+��Ag
+��NO
3-���л������Ϳ�ʴҺ��Ҫ�ȼ����������ԭFe
3+��Ag
+��
���Լ������ܽ����������
��5����������Һ�м���������������Ȼ�����
�������AgCl�м����ǰ���NH
2OH������ַ�Ӧ��ɵ������ǰ�������ΪN
2����ӦΪ��2AgCl+2NH
2OH=N
2��+2Ag+2H
2O+2HCl�����ݷ�Ӧ���㣻
���������⿼��������ˮ���Ӧ�ã��������õ���ȡ������������жϣ��Լ�ѡ���Ŀ�ģ����ӷ���ʽ��ѧ����ʽ��д�ķ������������������ǹؼ�����Ŀ�Ѷ��еȣ�

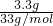 =0.1mol�����������ʵ���Ϊ0.1mol������Ϊ10.8g��
=0.1mol�����������ʵ���Ϊ0.1mol������Ϊ10.8g��

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�