
����Ŀ����1����֪CH3OH(l)��ȼ������H����238.6 kJ/mol��CH3OH(l)��3/2O2(g)===CO2(g)��2H2O(g)��H����a kJ/mol���� a___238.6(����>������<����������)��
��2��ʹCl2��H2O(g)ͨ�����ȵ�̿�㣬����HCl��CO2������1 mol Cl2���뷴Ӧʱ�ͷų�145 kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��_________________________________��
��3����ӦmA(g)+nB(g)![]() pC(g) +qD(g)�����е������仯��ͼ��ʾ���ش��������⡣
pC(g) +qD(g)�����е������仯��ͼ��ʾ���ش��������⡣
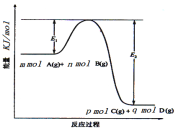
�÷�Ӧ��H =____(�ú�E1��E2ʽ�ӱ�ʾ)���ڷ�Ӧ��ϵ�м��������E1___��E2___��(������С������)��
��4����֪��CO (g) +H2O (g)![]() H2 (g) +CO2 (g)ƽ�ⳣ��K���¶ȵı仯���±���
H2 (g) +CO2 (g)ƽ�ⳣ��K���¶ȵı仯���±���
�¶�/�� | 400 | 500 | 800 |
ƽ�ⳣ��K | 9.94 | 9 | 1 |
�ش��������⣺
�ٸ÷�Ӧ����H=__________����>������=������<������
����֪��һ���¶��£�C(s) +CO2 (g)![]() 2CO��g)ƽ�ⳣ��K1��C (s) +H2O (g)
2CO��g)ƽ�ⳣ��K1��C (s) +H2O (g)![]() CO��g) +H2 (g)ƽ�ⳣ��K2����K��K1 ��K2֮��Ĺ�ϵ��__________________________��
CO��g) +H2 (g)ƽ�ⳣ��K2����K��K1 ��K2֮��Ĺ�ϵ��__________________________��
���𰸡���2Cl2(g)��2H2O(g)��C(s)===4HCl(g)��CO2(g)����H����290 kJ/mol ��H=-��E2-E1��kJ/mol��С��С��K=K2/K1
��������
(1)ȼ������1mol������ȫȼ�������ȶ�������ų����������״�ȼ������CO2(g)��H2O(g)��Ӧ�ö�Һ̬ˮΪ�ȶ���������ų�������С��ȼ���ȣ��ʴ�Ϊ������
(2)��1mol Cl2���뷴Ӧʱ�ͷų�145kJ������2mol������Ӧ����290kJ����Ӧ���Ȼ�ѧ����ʽΪ��2Cl2(g)+2H2O(g)+C(s)�T4HCl(g)+CO2(g)��H=-290kJmol-1���ʴ�Ϊ��2Cl2(g)+2H2O(g)+C(s)�T4HCl(g)+CO2(g)��H=-290kJmol-1 ��
(3)��ͼ���֪�÷�Ӧ��һ���������ߵķ�Ӧ�������������ȷ�Ӧ����H=��Ӧ����ܼ���-��������ܼ��ܣ�������H=E1-E2����������ı��˷�Ӧ��;�������ͷ�Ӧ����Ļ�ܣ�����E1��E2�ı仯�Ǽ�С���������ı䷴Ӧ����������������������֮���Ӧ�Ȳ��䣬����E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ����Ӱ�죬�ʴ�Ϊ��E1-E2����С����С��
(4)�ٸ��ݷ�ӦCO(g)+H2O(g)H2(g)+CO2(g)����ѧƽ�ⳣ������ʽ��K=![]() �������¶����ߣ��÷�Ӧ�Ļ�ѧƽ�ⳣ����С��ƽ�����������ƶ�������Ӧ�Ƿ��ȷ�Ӧ��������H��0���ʴ�Ϊ������
�������¶����ߣ��÷�Ӧ�Ļ�ѧƽ�ⳣ����С��ƽ�����������ƶ�������Ӧ�Ƿ��ȷ�Ӧ��������H��0���ʴ�Ϊ������
��C(s)+CO2(g)2CO(g)ƽ�ⳣ��K1����C(s)+H2O(g)CO(g)+H2(g)ƽ�ⳣ��K2����CO(g)+H2O(g)H2(g)+CO2(g)ƽ�ⳣ��K�����ݸ�˹���ɣ���=��-�٣���K=![]() ���ʴ�Ϊ��K=
���ʴ�Ϊ��K=![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£���������������Ϊ���淴ӦA��g��+3B��g��![]() 2C��g���ﵽƽ���־����
2C��g���ﵽƽ���־����
��C������������C�������������
�ڵ�λʱ��������a mol A��ͬʱ����3a mol B
��A��B��C��Ũ�Ȳ��ٱ仯
��C�����ʵ������ٱ仯
�ݻ���������ѹǿ���ٱ仯
�������������ʵ������ٱ仯
�ߵ�λʱ������a molA��ͬʱ����3a mol B
��A��B��C�ķ�����֮��Ϊ1��3��2
A. �ݢ�B. �ߢ�C. �٢�D. �ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ȱ��ά����A�������Ƥ�����ҹä֢��֢״��ά����A�ֳ��ӻƴ������ӽṹ����ͼ��ʾ,����˵����ȷ����
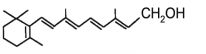
A. 1molά����A�������7molH2�����ӳɷ�Ӧ
B. ά����A���ܱ������õ�ȩ
C. ά����A��һ��������ˮ�Ĵ�
D. ά����A�ķ���ʽΪC20H30O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2��10- 5mol/LKCl ��2��10- 5mol/LAgNO3��Һ�������ϣ�����˵����ȷ���� (��֪AgCl ��Ksp=1.8��10-10 )
A.��AgCl��������B.��AgCl��������
C.��ȷ������AgCl��������D.�г���������AgCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������и������ʣ��� 126C��136C����ʯī������ϩ����Ư�ۺ�Ư��Һ��Ҫ�ɷ� �������������飻����ϩ�;���ϩ����![]() ��
�� ���߱���ͻ����� ��CH3-CH2-CH2-CH3 ��CH3-CH(CH3)CH3���������գ�
���߱���ͻ����� ��CH3-CH2-CH2-CH3 ��CH3-CH(CH3)CH3���������գ�
(1)____________������Ϊͬϵ�
(2)____________������Ϊͬ���칹��
(3)____________����������ͬλ�ء�
(4)____________������Ϊͬ�������塣
(5)____________��������ͬһ���ʡ�
��������ϩ�ڻ�����������Ӧ�ù㷺��
(1)��ϩ�ܺϳɺܶ���ʵ�ü�ֵ���л��

���Լ�a��_____________��
�ڷ�Ӧ��ķ�Ӧ������_____________��
(2)��ԭ��Ϊ��ʼԭ�Ϻϳɾ���ϩ��·������ͼ��ʾ��
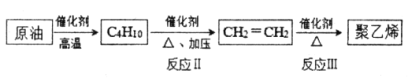
�ٷ�Ӧ��Ļ�ѧ����ʽ��_____________��
��д������ʽ����C4H10���л���Ľṹ��ʽ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����˵����ӦX(g)+2Y(g)![]() 2Z(g)�ﵽ��ѧƽ��״̬����
2Z(g)�ﵽ��ѧƽ��״̬����
A. X��Y��Z�����ʵ���֮��Ϊ1:2:2B. X��Y��Z��Ũ�Ȳ��ٷ����仯
C. ��Ӧ����v(X)= v(Y)D. ��λʱ��������n mol Z��ͬʱ����2n mol Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Һ�У���������һ���ܹ�������������� ��
A. ���������ܷų���������Һ�� Na+��Cl-��SO42-��Fe3+
B. ʹ��ɫʯ����Һ������Һ��Fe2+��Mg2+��NO3-��Cl-
C. c(OH��)=10-12 mol��L-1����Һ��K+��Ba2+��Cl-��Br-
D. ̼������Һ��K+��SO42-��Cl-��H+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijУѧϰС���ͬѧ����÷���ɸ��CH4��ԭNO2�����黹ԭ����ش��������⣺
��1������ͬѧ�������ͼ��ʾװ�÷ֱ��Ʊ�CH4��NO2��
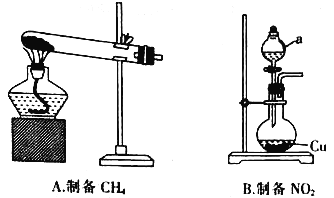
���ڼ��������£���ˮCH3 COONa��NaOH(CaOΪ����)����CH4��Na2CO3�Ļ�ѧ����ʽΪ___________��
������a������Ϊ__________������a�е�ҩƷ��__________(������)��__________(������������������)��������Բ����ƿ�е�ͭ��
��2������ͬѧ���ü����Ƶõĸ������岢��������װ����CH4��ԭNO2
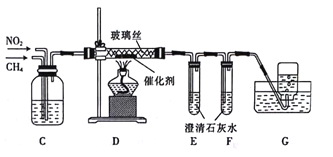
��Cװ�õ�������______________(�о�2��)��
��Dװ�÷�Ӧ����ʢ�Ų���˿��Ŀ����___________��CH4��NO2����N2��CO2�Ļ�ѧ����ʽΪ_____________��
��Eװ���г���ʯ��ˮδ����ǣ�Fװ���г���ʯ��ˮ����ǣ���ԭ�������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ԫ�����ʳ��������Ա仯�ĸ���ԭ����
A. ԭ�Ӱ뾶�������Ա仯
B. Ԫ�ػ��ϼ۳������Ա仯
C. ���Ӳ���������
D. Ԫ��ԭ�ӵĺ�������Ų��������Ա仯
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com