
��ij��ͭ��ʯ[��Ҫ�ɷ���FeCuSi3O13(OH)4��������SiO2��CaCO3]Ϊԭ���Ʊ�CuSO4��5H2O���������£�
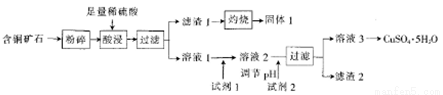
��֪����Լ��ɷֺͼ۸����±���ʾ��
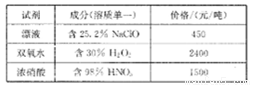
��ش��������⣺
��1����ͭ��ʯ�����Ŀ����_______��
��2����������Һ�г���Cu2+�⣬�����еĽ�����������_______��
��3������1����NaOH��Һ�����ӷ���ʽΪ__________��
��4�����������Ϣ��֪����ѡ�õ��Լ�1������Ϊ_______��������Լ�ʱ��������Ӧ�����ӷ���ʽΪ_________��
��5���Լ�2 ����ѡ����������е�______������2��һ�����е�����Ϊ______(�ѧʽ����
A. Cu B.CuO C.Cu(OH)2 D.Fe
��6��CuSO4��5H2O���ڵ�⾫��ͭʱ��������ͨ��9.632��103C�ĵ�������������ܽ��ͭΪ16.0g�����������Һ��ԭ��ҺΪ1 L����ǡ����CuSO4����������������������_____g��ԭ���Һ��CuSO4��Ũ��Ϊ__ ����֪һ�����ӵĵ���Ϊ1.6��10-19C��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����С������и������ϵ��п��������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ���ܴﵽ��ӦĿ�ĵ���
A. ���Ҵ���ȡ��ˮ�е��嵥��
B. �ø����pH��ֽ�ⶨ������ˮ��pH
C. ��NH4Cl��Һ�еμ�ʯ����Һ����Һ��죬֤��NH4Cl������ˮ�ⷴӦ
D. ������ˮ�еμӹ����ı���FeCl3��Һ�������Ͻ��裬��ȡFe(OH)3����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ��ͬ�и�һ3���¿���ѧ�Ծ��������棩 ���ͣ������
����Ԫ�����ڱ������ǿ�����ʶ����Ԫ�ص�����
��1��Ԫ�����ڱ�λ�ڶԽ��ߵ�����Ԫ�����������Ƴ�֮Ϊ�Խ��߹��������ڱ��Խ��߹��ɣ� ����Be���ʼ��仯����������������ʼ��仯������������ơ������˵Be(OH)2��Mg(OH)2��ѡ�õ��Լ�Ϊ__________��Һ��
��2��Ǧ(Pb)������Sn�����ࣨGe����Ԫ�أ�C)���裨Si��ͬ���壬�������䵥���ڿ����У��������Ӧ��Ǧ��������һ������Ǧ�����������Ӧ�����������ᷴӦ���ɴ˿ɵó����½��ۣ�
�� ���ԭ������Ϊ__________��
�� Ǧ��Pb��������Sn�����ࣨGe����+4����������ļ�����ǿ������˳��Ϊ__________���ѧʽ����
��3��������¹���ѧ��ʵ�����ԭ�����峬����̬���Ե̬�Ŀ���ת�����óɹ��������Ӽ�����о�����������ش�ͻ�ơ���֪﨣�Rb����37��Ԫ�أ���������85������ͬ���塣�ش��������⣺
��������ڱ��е�λ��Ϊ___________________
��ͬ����Ԫ�ص�ͬ�������������ƣ���д����
AlCl3�������RbOH��Ӧ�����ӷ���ʽ��_____________________________
������墨���һ�ּ�����γɵĻ�Ͻ���50g������������ˮ��Ӧʱ���ų���״���µ�����22��4L�����ּ����������__________
A��Li B��Na C��K D��Cs
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������ʡ����3���¿������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
������أ�K2FeO4����һ�����͡���Ч�����ˮ��������
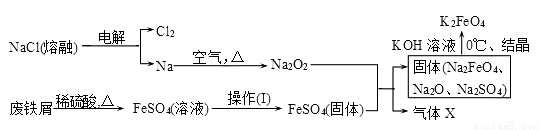
��1����ҵ�ϵ�ʪ���Ʊ���������KClO��Fe��OH��3��KOH�������Ƶ�K2FeO4���÷�Ӧ�������뻹ԭ�����ʵ���֮��Ϊ________________��
��2��ʵ������ʳ�Ρ�����м�����ᡢKOH��Ϊԭ�ϣ�ͨ�����¹����Ʊ�K2FeO4��
�ٲ������ķ���Ϊ_________________������������ѹ���
�ڼ������X����ķ�����________________��
����������Һ�еõ�K2FeO4�������õ�ԭ����____________________��
��3���ⶨijK2FeO4��Ʒ������������ʵ�鲽�����£�
����1��ȷ����1.0g��Ʒ������100mL��Һ��
����2��ȷ��ȡ25.00mL K2FeO4��Һ���뵽��ƿ��
����3����ǿ������Һ�У��ù���CrO2����FeO42����Ӧ����Fe��OH��3��CrO42��
����4����ϡ���ᣬʹCrO42��ת��ΪCr2O72����CrO2��ת��ΪCr3+��Fe��OH��3ת��ΪFe3+
����5�������������������ָʾ������0.1000mol��L��1 ��NH4��2Fe��SO4��2����Һ�ζ����յ㣨��Һ���Ϻ�ɫ�����������ģ�NH4��2Fe��SO4��2��Һ���������3��ƽ��ʵ�飬ƽ�����ģ�NH4��2Fe��SO4��2��Һ�����30.00 mL��
��֪���ζ�ʱ�����ķ�ӦΪ��6Fe2+��Cr2O72����14H+=6Fe3+��2Cr3+��7H2O��
�ٲ���2��ȷ��ȡ25.00mL K2FeO4��Һ���뵽��ƿ�����õ�������______________��
��д������3�з�����Ӧ�����ӷ���ʽ__________________________��
�۲���5���ܷ�ָʾ��_________��ԭ����________________��
�ܸ�������ʵ�����ݣ��ⶨ����Ʒ��K2FeO4����������Ϊ__________��
��4������0.1mol��L-1��K2FeO4��������ҺpH������������ˮ��Һ�еĴ�����̬��ͼ��ʾ������˵����ȷ����__________ ������ĸ����
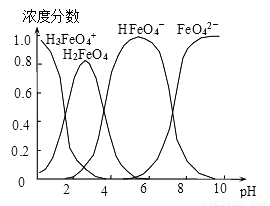
A��pH=2ʱ��c��H3FeO4+��+c��H2FeO4��+c��HFeO4����=0.1mol��L-1
B����pH��10��������Һ�м�����泥���HFeO4���ķֲ�����������
C����pH��1����Һ�м�HI��Һ��������Ӧ�����ӷ���ʽΪ��H2FeO4��H+��H3FeO4��
D����K2FeO4��������ˮ��ˮ��Һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������ʡ����3���¿������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��a��b��c��d����Ԫ�أ�ԭ��������������a����a+��a���������ӣ�b��cΪ������ͬһ����Ԫ�أ�c�Ĵ������8�����ӣ�c2����d2+�ĵ��Ӳ�ṹ��ͬ������������ȷ���ǣ� ��
A. b��c�ֱ���a�γɻ��������Է�������һ��Ϊb��c
B. a��d�γɵĻ�������ˮ��Ӧ�������������ȼ��
C. +6�۵�c��a��b�γ����ӻ�����
D. a��b��c��d����Ԫ����ɵĻ������ˮ��Һ��������Na2CO3ֻ�������壬����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ӱ�ʡ2017�������ѧ���������������ۺϻ�ѧ�Ծ� ���ͣ�ѡ����
��NA��ʾ����٤��������ֵ�����������в���ȷ����
A. 28g����ϩ�ͻ�����(C4H8)��ɵĻ�������к��е�̼ԭ����Ϊ2NA
B. �����£�1L0.5mol/LFeCl3��Һ�к��е�Fe3+��Ŀһ��С��0.5NA
C. 92g��NO2��N2O4��ɵĻ�������к��е�ԭ������Ϊ6NA
D. 22.4L����������þ�۳�ַ�Ӧ��ת�Ƶĵ�����Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ̫ԭ�и�һ3�½��Բ��Ի�ѧ�Ծ��������棩 ���ͣ������
���ֶ�����Ԫ�������ڱ��е����λ��������ʾ������ZԪ������������������Ӳ�����2������ش��������⣺
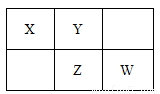
��1��Ԫ��Zλ�����ڱ��е�λ��_____________��
��2���ڱ�״���£��ó���XԪ�ص��⻯�����弰���������Ļ���������ƿ����Ȫʵ�飬ʵ�����������ƿ��������Һ�����ʵ���Ũ��Ϊ____________mol/L�����������λ��Ч���֣���
��3��W��Z������������Ӧ��ˮ��������Դ�С˳��____________���û�ѧʽ��ʾ����
��4����W�ĵ���ͨ�뺬�����ʵ���FeBr2����Һ�У�������Ӧ�����ӷ���ʽΪ
_______________________________________��
��5��Z��X�γɵ�һ�ֻ�������Է���������170��190֮�䣬��Z����������ԼΪ70�����û�����Ļ�ѧʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ̫ԭ�и�һ3�½��Բ��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
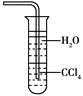
A. ��������Ũ�������Ҳ������ˮCaCl2����
B. NH3�ǵ���ʣ�����ˮ�ܵ���
C. NH3����ʹ����ĺ�ɫʯ����ֽ����
D. ��ˮ����NH3����ͼװ�ÿɷ�ֹ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�����и�һ��ѧ�ڿ�ѧ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��200mL�Ȼ�þ���Ȼ����Ļ����Һ������C��Mg2����Ϊ0.2mol��L��1��C��Cl����Ϊ1.3mol��L-1��ҪʹMg2��ȫ��ת��Ϊ��������������������4mol��L��1NaOH��Һ�����Ϊ
A. 80mL B. 40mL C. 72mL D. 128mL
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com