
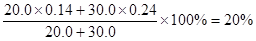
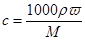 æÉÖŖ»ģŗĻŗóČÜŅŗÖŠNaClµÄĪļÖŹµÄĮæÅØ¶ČŹĒ
æÉÖŖ»ģŗĻŗóČÜŅŗÖŠNaClµÄĪļÖŹµÄĮæÅØ¶ČŹĒ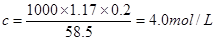
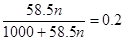 £¬½āµĆx£½4.3mol
£¬½āµĆx£½4.3mol

 ĆūŹ¦°éÄć³É³¤æĪŹ±Ķ¬²½Ń§Į·²āĻµĮŠ“š°ø
ĆūŹ¦°éÄć³É³¤æĪŹ±Ķ¬²½Ń§Į·²āĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£® mol mol | B£®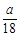 mol mol | C£® mol mol | D£® mol mol |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®±ź×¼×“æöĻĀ£¬11.2 Lŗ¤ĘųÖŠŗ¬ÓŠ0.5 NAŌ×Ó |
| B£®±ź×¼×“æöĻĀ£¬0.1 mol Cl2²Ī¼Ó·“Ó¦£¬×ŖŅʵĵē×ÓŹżÄæŅ»¶ØĪŖ0. 2 NA |
| C£®³£ĪĀ³£Ń¹ĻĀ£¬46g NO2ŗĶN2O4»ģŗĻĘųĢåÖŠŗ¬ÓŠŌ×Ó×ÜŹżĪŖ3NA |
| D£®1 mol NaÓė×ćĮæO2·“Ó¦£¬Éś³ÉNa2OŗĶNa2O2µÄ»ģŗĻĪļ£¬×ŖŅʵē×Ó×ÜŹżNAøö |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®øĆŌ×ÓµÄĦ¶ūÖŹĮæŹĒaNA |
B£®WgøĆŌ×ÓµÄĪļÖŹµÄĮæŅ»¶ØŹĒ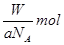 |
| C£®øĆŌ×ÓµÄĻą¶ŌŌ×ÓÖŹĮæŹĒ12a/b |
| D£®ÓÉŅŃÖŖŠÅĻ¢æÉµĆ£ŗ NA=12/b |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®22.4 L NH3ÖŠŗ¬µŖŌ×ÓŹżĪŖ NA |
| B£®1 mol Na2O2ÓėĖ®ĶźČ«·“Ó¦Ź±×ŖŅʵĵē×ÓŹżĪŖNA |
| C£®100 mL 2.0 mol”¤L-1 NH4HCO3ČÜŅŗÖŠNH4+ ŹżĪŖ0.2NA |
| D£®1 mol O2ŗĶ2 mol SO2ŌŚĆܱÕČŻĘ÷ÖŠ³ä·Ö·“Ó¦ŗóµÄ·Ö×ÓŹżµČÓŚ2NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®28g N2Ėłŗ¬ÓŠµÄŌ×ÓŹżĪŖNA |
| B£®1.8gµÄNH4£«Ąė×ÓÖŠŗ¬ÓŠµē×ÓŹż11NA |
| C£®22.4LCH4µÄÖŹĮæÓėNAøö¼×Ķé·Ö×ÓµÄÖŹĮæÖ®ŗĶĻąµČ |
| D£®±ź×¼×“æöĻĀ£¬22.4LCH4ŗĶO2µÄ»ģŗĻĘųĢåĖłŗ¬ÓŠµÄ·Ö×ÓŹżĪŖNA |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com