
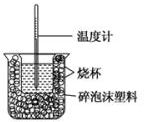
 ŅŃÖŖŌŚĻ”ČÜŅŗÖŠ£¬Ėįøś¼ī·¢ÉśÖŠŗĶ·“Ӧɜ³É1molĖ®Ź±µÄ·“Ó¦ČČ½Š×öÖŠŗĶČČ£®ĻÖĄūÓĆČēĶ¼×°ÖĆ½ųŠŠÖŠŗĶČČµÄ²ā¶Ø£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ŅŃÖŖŌŚĻ”ČÜŅŗÖŠ£¬Ėįøś¼ī·¢ÉśÖŠŗĶ·“Ӧɜ³É1molĖ®Ź±µÄ·“Ó¦ČČ½Š×öÖŠŗĶČČ£®ĻÖĄūÓĆČēĶ¼×°ÖĆ½ųŠŠÖŠŗĶČČµÄ²ā¶Ø£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ·ÖĪö £Ø1£©øł¾ŻĮæČČ¼ĘµÄ¹¹ŌģĄ“ÅŠ¶ĻøĆ×°ÖƵÄȱɣµÄ²æ·Ö£»
£Ø2£©ÖŠŗĶČČ²ā¶ØŹµŃé³É°ÜµÄ¹Ų¼üŹĒ±£ĪĀ¹¤×÷£»
£Ø3£©²Ł×÷Ź±·Ö¼ø“Ī×¢Čė·“Ó¦Ņŗ£¬µ¼ÖĀČČĮæÉ¢Ź§£»
£Ø4£©ÖŠŗĶČČ²ā¶ØŹµŃéÖŠŠčŅŖĪĀ¶Č¼Ę²āĮæĖį”¢¼īŗĶ·“Ó¦ŗóµÄ×īøßĪĀ¶ČČż“Ī£»
£Ø5£©ĻČøł¾ŻQ=m•c•”÷T¼ĘĖć·“Ó¦·Å³öµÄČČĮæ£¬Č»ŗóøł¾Ż”÷H=-$\frac{Q}{n}$kJ/mol¼ĘĖć³ö·“Ó¦ČČ£»
£Ø6£©ĄūÓĆøĒĖ¹¶ØĀÉĄ“¼ĘĖć·“Ó¦ģŹ±ä²¢ŹéŠ“ČČ»Æѧ·½³ĢŹ½£®
½ā“š ½ā£ŗ£Ø1£©ÓÉĮæČČ¼ĘµÄ¹¹ŌģæÉÖŖøĆ×°ÖƵÄȱɣŅĒĘ÷ŹĒ»·ŠĪ²£Į§½Į°č°ōŗĶÉÕ±ÉĻ·½µÄÅŻÄĖÜĮĻøĒ£»
¹Ź“š°øĪŖ£ŗ»·ŠĪ²£Į§½Į°č°ō£»ÉÕ±ÉĻ·½µÄÅŻÄĖÜĮĻøĒ£»
£Ø2£©ÖŠŗĶČČ²ā¶ØŹµŃé³É°ÜµÄ¹Ų¼üŹĒ±£ĪĀ¹¤×÷£¬“óŠ”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄĖÜĮĻµÄ×÷ÓĆŹĒ¼õÉŁŹµŃé¹ż³ĢÖŠµÄČČĮæĖšŹ§£»
¹Ź“š°øĪŖ£ŗ¼õÉŁŹµŃé¹ż³ĢÖŠµÄČČĮæĖšŹ§£»
£Ø3£©²Ł×÷Ź±·Ö¼ø“Ī×¢Čė·“Ó¦Ņŗ£¬µ¼ÖĀČČĮæÉ¢Ź§£¬ĒóµĆµÄÖŠŗĶČČŹżÖµ½«»į¼õŠ”£»
¹Ź“š°øĪŖ£ŗĘ«Š”£»
£Ø4£©ÖŠŗĶČČ²ā¶ØŹµŃéÖŠŠčŅŖĪĀ¶Č¼Ę²āĮæĖį”¢¼īŗĶ·“Ó¦ŗóµÄ×īøßĪĀ¶ČČż“Ī£¬ĖłŅŌÖĮÉŁŠčŅŖŹ¹ÓĆĪĀ¶Č¼Ę3“Ī£»
¹Ź“š°øĪŖ£ŗ3£»
£Ø5£©ĘšŹ¼Ę½¾łĪĀ¶ČĪŖt1”ę£¬»ģŗĻ·“Ó¦ŗó×īøßĪĀ¶ČĪŖt2”ę£¬ĪĀ¶Č²īĪŖ£ŗ£Øt2-t1£©”ę£¬0.5mol/LµÄŃĪĖįŗĶ0.55mol/LµÄNaOHČÜŅŗø÷50mL½ųŠŠŹµŃé£¬Éś³ÉĖ®µÄĪļÖŹµÄĮæĪŖ0.05L”Į0.50mol=0.025mol£¬ČÜŅŗµÄÖŹĮæĪŖ£ŗ100ml”Į1g/cm3=100g£¬ŌņÉś³É0.025molĖ®·Å³öµÄČČĮæĪŖQ=m•c•”÷T=100g”Į4.18”Į10-3kJ/£Øg•”ę£©”Į£Øt2-t1£©”ę£¬ĖłŅŌŹµŃé²āµĆµÄÖŠŗĶČČ”÷H=-$\frac{100g”Į4.18”Į1{0}^{-3}kJ/£Øg•”ę£©”Į£Øt2-t1£©”ę}{0.025mol}$=-$\frac{0.418£Ø{t}_{2}-{t}_{1}£©}{0.025}$kJ/mol£»
¹Ź“š°øĪŖ£ŗ-$\frac{0.418£Ø{t}_{2}-{t}_{1}£©}{0.025}$£»
£Ø6£©HCl£Øaq£©+NaOH£Øaq£©ØTNaCl£Øaq£©+H2O£Øl£©”÷H1=a kJ/mol ¢Ł
HCl£Øaq£©+NH3•H2O£Øaq£©ØTNH4Cl£Øaq£©+H2O£Øl£©”÷H2=b kJ/mol ¢Ś
ÓÉøĒĖ¹¶ØĀÉ¢Ś-¢ŁµĆ£ŗNH3•H2O£Øaq£©?NH4+£Øaq£©+OH-£Øaq£©”÷H3=£Øb-a£©kJ/mol£»
¹Ź“š°øĪŖ£ŗ£Øb-a£©£®
µćĘĄ ±¾Ģāæ¼²éÖŠŗĶČČµÄ²ā¶ØŌĄķÓė¼ĘĖć”¢øĒĖ¹¶ØĀɵÄŌĖÓĆ£¬ĢāÄæÄѶČÖŠµČ£¬×¢ŅāĄķ½āÖŠŗĶČȵÄøÅÄīÓėŌĄķŹĒ½āĢāµÄ¹Ų¼ü£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
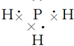 £¬½į¹¹Ź½
£¬½į¹¹Ź½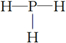 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1øö | B£® | 2øö | C£® | 3øö | D£® | 4øö |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
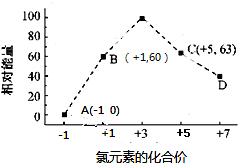
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĆŗĢæ¾Ęų»Æ”¢Ņŗ»ÆŗĶøÉĮóµČ¹ż³Ģ£¬æÉ»ńµĆĒå½ąÄÜŌ“ŗĶÖŲŅŖµÄ»Æ¹¤ŌĮĻ | |
| B£® | »ś¶Æ³µŹµŠŠĻŽŠŠ“ėŹ©ŹĒ¼õÉŁĪķö²µÄĶ¾¾¶Ö®Ņ» | |
| C£® | ČÕ³£Éś»īÖŠČĖĆĒ“óĮæŹ¹ÓĆĀĮÖĘĘ·£¬ŹĒŅņĪŖ³£ĪĀĻĀĀĮ²»ÄÜÓėŃõĘų·“Ó¦ | |
| D£® | ÉńÖŪ10ŗÅ·É“¬ĖłÓĆĢ«ŃōÄܵē³Ų°åæɽ«¹āÄÜ×Ŗ»»ĪŖµēÄÜ£¬ĖłÓĆ×Ŗ»»²ÄĮĻŹĒµ„¾§¹č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | 0.1molōĒ»łÖŠŗ¬ÓŠ1 molµē×Ó | |
| B£® | ŅŅĶéŗĶ±ūĻ©ČĪŅā±Č»ģŗĻµÄĘųĢå1mol£¬Ķź³ÉČ¼ÉÕ¾łÉś³É3molH2O | |
| C£® | CH2Cl2¾ßÓŠĮ½ÖÖĶ¬·ÖŅģ¹¹Ģå | |
| D£® | Ķ¼ÖŠÓŠ»śĪļ£Ø1-¼×»ł»·¼ŗĶ飩µÄŅ»ĀČ“śĪļÓŠ4ÖÖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CaO | B£® | CaCl2 | C£® | NaOH | D£® | C2H6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ½µµĶĪĀ¶Č | B£® | ¼ÓČėŹŹĮæµÄĖ® | ||
| C£® | ¼ÓČėÉŁĮæCuSO4ČÜŅŗ | D£® | ¼ÓČėÅØ¶Č½Ļ“óµÄŃĪĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ·ÅČČ·“Ó¦µÄ·“Ó¦ĖŁĀŹŅ»¶Ø“óÓŚĪüČČ·“Ó¦µÄ·“Ó¦ĖŁĀŹ | |
| B£® | ČŪȌדĢ¬ĻĀÄܵ¼µēµÄ»ÆŗĻĪļŅ»¶Øŗ¬Ąė×Ó¼ü | |
| C£® | Ōö“ó·“Ó¦ĪļÅضČæɼÓæģ·“Ó¦ĖŁĀŹ£¬Ņņ“ĖæÉÓĆÅØĮņĖįÓėŠæ·“Ó¦Ōö“óÉś³ÉĒāĘųµÄĖŁĀŹ | |
| D£® | ŹĒ·ńÓŠ¶”“ļ¶ūĻÖĻóŹĒ½ŗĢåŗĶČÜŅŗµÄ±¾ÖŹĒų±š |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com