
�ҹ����ֳ��л�����ռȫ��һ�룬���������PM2.5ϸ���Ӱ�����NH4��2SO4��NH4NO3���л������P�ﳾ�ȡ�ͨ���ⶨ������п���ؽ����ĺ�������֪Ŀǰ����ҹ�����������Ҫ�ǽ�ͨ��Ⱦ��
��1��Zn2+�ڻ�̬ʱ��������Ų�ʽΪ____ _��
��2��SO42-�Ŀռ乹����___ __(����������)��
��3��PM2.5�����������ж����к����ʣ����������ι⻯ѧ������Ⱦ���⻯ѧ�����к���NOX��O3��CH2=CH-CHO, HCOOH, CH3COOONO2(PAN)�ȶ�����Ⱦ�
������˵����ȷ����___ __(������ѡ��)��
a��N2O�ṹʽ�ɱ�ʾΪN=N=O
b��O3���ӳ�ֱ����
c��CH2=CH-CHO������̼ԭ�Ӿ�����sp2�ӻ�
d����ͬѹǿ�£�HCOOH�е��CH3OCH3�ߣ�˵��ǰ���Ǽ��Է��ӣ������ǷǼ��Է���
��1mo1PAN�к��Ҽ���ĿΪ_____��
��NO�ܱ�FeS04��Һ�������������[(Fe(NO)(H20)5)S04,��������������ӵ���λ��Ϊ_____(������)��
��4���ⶨ������PM2.5��Ũ�ȷ���֮һ�Ǧ�һ�������շ�����һ���߷���Դ����85Kr����֪Kr����ľ����ṹ��ͼ��ʾ���辧������ÿ��Krԭ������ڵ�Krԭ����m���������к�Krԭ��Ϊn������m/n=_____(������)��

��1��1s22s22p63s23p63d10��[Ar]3d10
��2����������
��3����ac
��10mol(��10��6.02��1023��6.02��1022)
��6
��4��3
��������
�����������1��ZnΪ30�ţ�Zn2����ʧȥ4s�����2�����ӡ���2��SO42 ���У�S�¶Ե��ӣ�S��O�γ��ĸ���ѧ������SΪsp3�ӻ��������������Ρ���3����N2O��CO2��Ϊ�ȵ����壬N��O֮��Ϊ˫����a��ȷ��O3��SO2��Ϊ�ȵ����壬ΪV�η��ӣ�b����CH2=CH-CHO��ÿ��̼ԭ�Ӿ��൱���γ����������ʾ�Ϊsp2�ӻ���c��ȷ��HCOOH���Ӽ�����γ�������ʷе�ߣ�d����PAN�����е�����Ϊ�Ҽ���˫������һ��Ϊ�Ҽ���-NO2�ĽṹΪ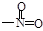 ��������λ�ڷ������У���1��NO��5��H2O���ɣ�����λ��Ϊ6����4���Զ�����㣬��֮���������Krλ�����������ϣ��������ԭ��Ϊ8�������干�У�ÿ�������ϵ�KrΪ���������干�У��������KrΪ3��8/2=12��������Kr��8��1/8+6��1/2=4�����߱�ֵΪ12��4=3��
��������λ�ڷ������У���1��NO��5��H2O���ɣ�����λ��Ϊ6����4���Զ�����㣬��֮���������Krλ�����������ϣ��������ԭ��Ϊ8�������干�У�ÿ�������ϵ�KrΪ���������干�У��������KrΪ3��8/2=12��������Kr��8��1/8+6��1/2=4�����߱�ֵΪ12��4=3��
���㣺���ʽṹ�����ӽṹ������ṹ����ؼ���


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
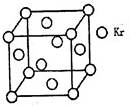 �����ʽṹ�����ʡ�
�����ʽṹ�����ʡ�| m | n |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com