
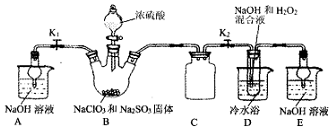
���� ��ȡNaClO2���壺װ��B�з�����Ӧ��2NaClO3+Na2SO3+H2SO4=2ClO2��+2Na2SO4+H2O�����ɵ�ClO2���徭װ��C����װ��D��������Ӧ��2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2����NaClO2��Һ���������ᾧ�����ˡ�ϴ�ӡ�����Ȳ������þ���NaClO2•3H2O��Ҫע�������Ŀ������Ϣ�������¶ȣ�װ��AE�����ն��������ֹ��Ⱦ���ݴ˷�������
��� �⣺��1��װ��B��ʹŨ����˳�����£�����Һ©������������©���Ͽڲ�С�����ٿ�����Һ©����������װ�����������壬��ѧ����ʽΪ��2NaClO3+Na2SO3+H2SO4=2ClO2��+2Na2SO4+H2O��
�ʴ�Ϊ������Һ©������������©���Ͽڲ�С�����ٿ�����Һ©��������2NaClO3+Na2SO3+H2SO4=2ClO2��+2Na2SO4+H2O��
��2��ClO2��Ⱦ�����������ŷ��ڿ����У�װ��AE������ClO2����ֹ��Ⱦ������
�ʴ�Ϊ������ClO2����ֹ��Ⱦ������
��3��װ��D��ΪClO2�������������ơ��������ⷴӦ����NaClO2�ķ�Ӧ����Ԫ�ػ��ϼ۽��ͣ��������������Ԫ�ػ��ϼ�����������������ѧ����ʽΪ��2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2��
�ʴ�Ϊ��2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2��
��4����ΪNaClO2������Һ���¶ȵ���38��ʱ�����ľ�����NaClO2•3H2O������38��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl����Ҫ�õ�NaClO2���壬����38-60��õ����壬�ʲ���Ϊ��װ��D��Ӧ�����Һ��55�������¼�ѹ�����ᾧ�����ȹ��ˣ�����38�桫60�����ˮϴ�ӣ�����ڵ���60�������¸���õ�NaClO2���壻
�ʴ�Ϊ�����ȹ��ˣ�
��5��������������������������������������ӣ����ӷ���ʽΪ��ClO2-+4I-+4H+=2H2O+2I2+Cl-��
�ʴ�Ϊ��ClO2-+4I-+4H+=2H2O+2I2+Cl-��
��6���еⵥ�����ɺ����ģ��õ�����Һ��ָʾ����
�ʴ�Ϊ��������Һ��
��7������Ʒ��NaClO2����������Ϊx����
NaClO2��2I2��4S2O32-
90.5g 4mol
axg b mol•L-1��V��10-3L��$\frac{100mL}{25mL}$��
����90.5g��axg=4mol��b mol•L-1��V��10-3L��$\frac{100mL}{25mL}$��
���x=$\frac{0.0905bV}{a}$��
�ʴ�Ϊ��$\frac{0.0905bV}{a}$��
���� ���⿼�����ʵ��Ʊ�������Ϣ�����á���װ�õ����⡢������ԭ��Ӧ�ζ��ȣ�����ԭ���ǽ���Ĺؼ���ͬʱ����ѧ���������⡢����������������Ŀ�Ѷ��еȣ�ע��������ԭ��Ӧ�ζ������ù�ϵʽ���еļ��㣮


 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A | B | C | D | |
| ���� | CCl4 | H2SO4��Ũ�� | KClO3 | P4 |
| Σ�վ����ǩ |  |  |  |  |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | �ۢ� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʳ���ܻ� | B�� | �ɱ����� | ||
| C�� | �Ȼ�����ȣ�������ʧ | D�� | ʯī�ۻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
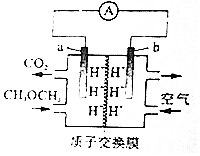
| A�� | a��������Һ��pH��� | |
| B�� | b����H2O���� | |
| C�� | a����Ӧʽ��CH3OCH3+3O2--12e-�T2CO2��+6H+ | |
| D�� | ÿ����11.2LO2����״��������2molH+������ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com