
| A����0.1mol?L-1NaHCO3��Һ�У�c��Na+����c��HCO3-����c��CO32-����c��H2CO3�� |
| B����0.1mol?L-1Na2CO3��Һ�У�c��OH-��-c��H+���Tc��HCO4-��+2c��H2CO3�� |
| C����0.2mol?L-1NaHCO3��Һ�м�������0.1mol?L-1NaOH��Һ��c��CO32-����c��HCO3-����c��OH-����c��H+�� |
| D�������£�pH=7��CH3COONa��CH3COOH�����Һ��c��Na+��+c��CH3COO-���Tc��H+��+c��OH-�� |


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��HCl |
| B��MgSO4 |
| C��CuSO4 |
| D��NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
. |
| M |
A��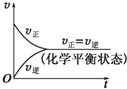 |
B��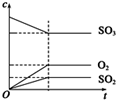 |
C�� |
D�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 4 |
| 3 |
| 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ũ���ᡢŨ���ᶼ����ǿ�����ԣ�����ʢ���ڽ��������� |
| B��Na2SiO3���Ʊ��轺��ľ�ķ������ԭ�� |
| C��ʳ�ο�����ζ���������������� |
| D���Ͻ��Ӳ�ȴ��۵�Ҳ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ʵ���������HA�������KA�Ļ����Һ�У�2c��K+��=c��HA��+c��A-�� |
| B��pH��ͬ��CH3COONa��Һ��Ba��OH��2��Һ��KHCO3��Һ��c��K+����c��Na+����c��Ba2+�� |
| C����ˮ����μ�������õ���������Һ��c��Cl-����c��NH4+����c��H+����c��OH-�� |
| D��pH=3��һԪ��HA��Һ��pH=11��һԪ��MOH��Һ�������ϣ�c��M+��=c��A-����c��H+��=c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����Ȼ�����Һ�м�������Fe3++Fe�T2Fe2+ |
B���������Ƶ���ʽ��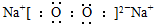 |
C��Na+�ṹ��ͼ��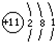 |
| D����������ͨ���廯������Һ��2FeBr2+3Cl2�T2FeCl3+2 Br2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
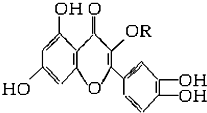 ��Ȼά����P���ṹ��ͼ�����ӽṹ��RΪ���������������ڻ��������У�����һ��Ӫ��������������ά����P��������ȷ���ǣ�������
��Ȼά����P���ṹ��ͼ�����ӽṹ��RΪ���������������ڻ��������У�����һ��Ӫ��������������ά����P��������ȷ���ǣ�������| A��������ˮ��Ӧ����1mol��������������ˮ��Ӧ����6molBr2 |
| B������NaOH��Һ��Ӧ��1mol�����ʿ���5molNaOH��Ӧ |
| C��һ��������1mol�����ʿ���H2�ӳɣ���H2�����Ϊ6mol |
| D��ά����P�ܷ���ˮ�ⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����֪�����ۻ���Ϊ6.0 kJ/mol�������������Ϊ20 kJ/mol������1 mol������2 mol ��������ۻ�����ȫ�����ƻ���������������ֻ���ƻ�����15%����� |
| B��SO2��SO3���Ǽ��Է��� |
| C��ʵ���û����飨l��������ϩ��l���ͱ���l���ı�ȼ���ȷֱ�Ϊ-3916 kJ/mol��-3747 kJ/mol��-3265 kJ/mol������֤���ڱ������в����ڶ�����̼̼˫�� |
| D���������еĹ����ǽ������չ��Ӷ�����ԾǨ�����ض������ĹⲨ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com