



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
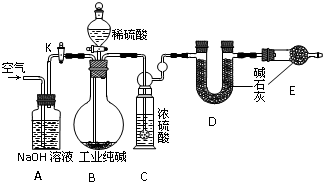
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
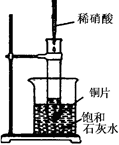
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��AlCl3��NaOH | B��NaHCO3��NaOH |
| C��Na2SO4��BaCl2 | D��AgNO3��NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
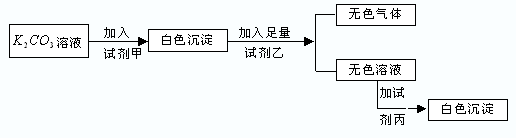
| �� | �� | �� |
| A��MgCl2 | HNO3 | K2SO4 |
| B��BaCl2 | HNO3 | K2SO4 |
| C��NaNO3 | H2SO4 | Ba��NO3��2 |
| D��BaCl2 | HCl | CaCl2 |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͨ��������������Ȼ�̼��Һ |
| B��ͨ����������ˮ |
| C����ͨ�����������Ը��������Һ����ͨ����ʯ�� |
| D�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
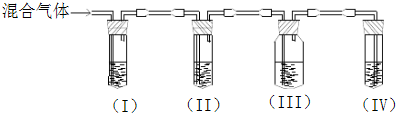
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ܢ٢ڢ� | B���٢ܢۢ� | C���٢ۢܢ� | D���ۢڢ٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ʵ��Ҫ�� | �Լ������� | ��ѡ��Ļ�ѧ�Լ���ʵ�鷽�� A��ͨ����ˮ��ϴ��ƿ B������ C��ͨ��ʢ����NaHCO3��Һ��ϴ��ƿ D������Һ�е����̪��Һ E���ܽ⡢���ˡ��ᾧ |
| �����Ȼ��ơ�̼��ƹ������� | ||
| ֤��������Һ�ʼ��� | ||
| ��ȥʳ���еĵⵥ�� | ||
| ��ȥCO2�������Ȼ��� | ||
| �ж���CH4�Ƿ���C2H2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com