
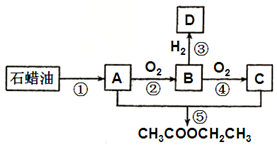
 ��A��һ����Ҫ�Ļ���ԭ�ϣ���֪A�ڱ�״���µ��ܶ�Ϊ1.25g•L-1��B�ɷ���������Ӧ������֮���ת����ϵ��ͼ��
��A��һ����Ҫ�Ļ���ԭ�ϣ���֪A�ڱ�״���µ��ܶ�Ϊ1.25g•L-1��B�ɷ���������Ӧ������֮���ת����ϵ��ͼ������ A�ڱ�״���µ��ܶ�Ϊ1.25g•L-1����A��Ħ������Ϊ1.25g/L��22.4L/mol=28g/mol��B�ɷ���������Ӧ����BΪȩ��������ͼ��֪��A��������B��B��������C��A��C������Ӧ����������������AΪCH2=CH2��BΪCH3CHO��CΪCH3COOH��DΪCH3CH2OH���Դ������
��� �⣺��1��������������֪��BΪCH3CHO������������Ϊȩ�����ʴ�Ϊ��ȩ����
��2��������������֪��AΪCH2=CH2��CΪCH3COOH���ڢݲ�Ϊ�����ӳɷ�Ӧ��A��C�ӳ�����������������Ӧ�Ļ�ѧ����ʽ�ǣ�CH2=CH2+CH3COOH$\stackrel{����}{��}$CH3COOCH2CH3���ʴ�Ϊ��CH2=CH2+CH3COOH$\stackrel{����}{��}$CH3COOCH2CH3��
��3��������������֪��DΪCH3CH2OH�������������л���D��CH3CH2OH��Ӧ����CH3CH2ONa��CH3CH2ONa����ˮ����ˮ��Ӧ�����Ҵ����������ƣ����Եμ�2�η�̪��Һ��ˮ��Һ�Ժ�ɫ����Ӧ����ʽΪCH3CH2ONa+H2O��CH3CH2OH+NaOH���ʴ�Ϊ��CH3CH2ONa+H2O��CH3CH2OH+NaOH��
��4��A��AΪCH2=CH2��DΪCH3CH2OH��CH3CH2OH�ܷ������Ӽ���ˮ�������ѣ����л���A��D��һ�������¿ɷ�Ӧ��������[��CH3CH2��2O]����A��ȷ��
B��BΪCH3CHO��CΪCH3COOH��DΪCH3CH2OH���������Ƽ���������ͭ����Һ������Һ�ܽ����ɫ��ҺΪCH3COOH���������ΪCH3CHO��CH3CH2OH���ټ�����ש��ɫ�������ɵ�ΪCH3CHO�������ܼ��𣬹�B����
C��CΪCH3COOH�����������������е����������������룬��C����
D����������������������Һ��ˮ�����������ƺ��Ҵ��������棬����ϡ�����и���ȫ����D��ȷ��
�ʴ�Ϊ��AD��
���� ���⿼���л�����ƶϣ�Ϊ��Ƶ���㣬������Ϣ�������ŵı仯Ϊ���Ĺؼ������ط������ƶ������Ŀ��飬ע��AΪ��ϩ����Ŀ�ѶȲ���


 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 3O3$\frac{\underline{\;�ŵ�\;}}{\;}$2O3 | B�� | 2KClO3 $\frac{\underline{MnO_2}}{��}$2KCl+3O2�� | ||
| C�� | Cl2+2NaOH�TNaCl+NaClO+H2O | D�� | CO+CuO $\frac{\underline{\;\;��\;\;}}{\;}$ Cu+CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2H2��g��+O2��g���T2H2O��l������H1 2H2��g��+O2��g���T2H2O��g������H2 | |
| B�� | S��g��+O2��g���T2SO2��g������H1 S��s��+O2��g���T2SO2��g������H2 | |
| C�� | C��s��+$\frac{1}{2}$ O2��g���TCO��g������H1 C��s��+O2��g���TCO2��g������H2 | |
| D�� | H2��g��+Cl2��g���T2HCl��g������H1 $\frac{1}{2}$H2��g��+$\frac{1}{2}$ Cl2��g���THCl��g������H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol������������������Ӧת�Ƶĵ�����ΪNA | |
| B�� | ��״���£�22.4L�ױ��к�C-H����ĿΪ8NA | |
| C�� | ��״���£�11.2L�����к���0.5NA����ԭ�� | |
| D�� | ���³�ѹ�£�����NA����������ϩ���ӵĻ����������Ϊ28g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO2���������ԣ�������Ư��ֽ�� | |
| B�� | NaHCO3����Ӧ�����������Ƹ������ɼ� | |
| C�� | Fe2��SO4��3������ˮ����������ˮ�� | |
| D�� | Һ������ʱ���մ������ȣ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʹ���ȱ��ɫ����Һ�У� K+��Ba2+��AlO2-��NO3- | |
| B�� | 1.0mol/L��KI��Һ�У�Na+��Fe3+��Cl-��SO42- | |
| C�� | ������Ӧ����������������Һ�У�Mg2+��HCO3-��Cl-��NO3- | |
| D�� | c��H+��=1��10-13mol/L��Һ�У�Na+��AlO2-��SiO32-��PO43- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com