
���� ��1������ʵ������IJ��裨������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ���Լ�ÿ��������Ҫ����ȷ����Ӧ��������������
��2������n=cV��������Na2CO3�����ʵ���������Na2CO3•10H2O�����ʵ�������Na2CO3�����ʵ�����m=nM����Na2CO3•10H2O��������
��3������c=$\frac{n}{V}$���㲻��������n��V��Ӱ�죬���nƫ���VƫС������������ҺŨ��ƫ�ߣ�
��� �⣺��1��������˳���ǣ�������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��һ������ƽ�������õ�ҩ�ף����������ձ����ܽ⣬��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ���ȱ���ձ���100mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ���ձ���100mL����ƿ����ͷ�ιܣ�
��2����Na2CO3�����ʵ���n=cV=0.1L��1.0mol•L-1=0.1mol��Na2CO3•10H2O�����ʵ�������Na2CO3�����ʵ���������Na2CO3•10H2O������0.1mol��286g/mol=28.6g��
�ʴ�Ϊ��28.6��
��3����ֻҪ�����ʱʹ��Һ����̶������м��ɣ�ԭ������ƿ������������ˮ������Һ�����Ӱ�죬�����ҺŨ����Ӱ�죬�ʴ�Ϊ��c��
���ձ��Ͳ���û��ϴ��2-3�Σ���������ʵ���ʧ��������ҺŨ��ƫ�ͣ��ʴ�Ϊ��b��
�����Ƶ���Һװ��ྻ�ĵ�����������ˮ���Լ�ƿ�У���������õ���Һ���ϡ�ͣ���Ũ��ƫ�ͣ��ʴ�Ϊ��b��
��������ʱ���ӣ��ᵼ����Һ���ƫС����������Һ�����ʵ���Ũ��ƫ�ߣ��ʴ�Ϊ��a��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ��̡������Լ����������ѶȲ���ע��ʵ��Ļ�������������ע�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
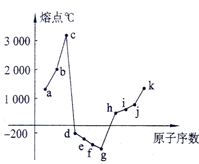 ��1-18��Ԫ���У���������Ԫ�ص��ʵ��۵������ͼ��ʾ���Իش�
��1-18��Ԫ���У���������Ԫ�ص��ʵ��۵������ͼ��ʾ���Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C2H6��C4H10һ����ͬϵ�� | |
| B�� | C2H4��C4H8һ����ͬϵ�� | |
| C�� | C3H6��ֻ��ʾһ������ | |
| D�� | ϩ���и�ͬϵ����̼������������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ҵ� | B�� | ���� | C�� | �������� | D�� | �״� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��ɫ�� | B�� | ������ | C�� | Ӫ��ǿ���� | D�� | ��ζ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
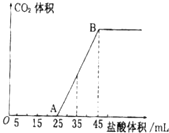 ʵ����������0.1mol/L NaOH��Һ�������й�����ʵ�飬��ݴ˻ش���������
ʵ����������0.1mol/L NaOH��Һ�������й�����ʵ�飬��ݴ˻ش����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ӦAl-3e-Al3+ | B�� | ȼ������������ | ||
| C�� | ������ӦΪ2CO2+O2+4e-2CO32- | D�� | �õ�ز���������������ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
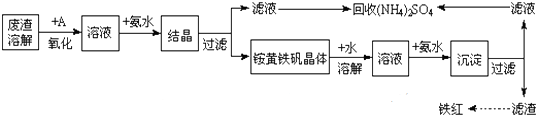
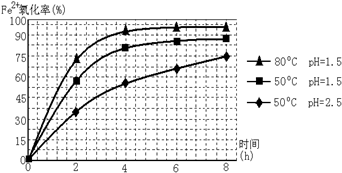
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH2OH$��_{170��}^{Ũ����}$CH2�TCH2��+H2O | |
| B�� | CH3CHBrCH3+NaOH$��_{��}^{�Ҵ�}$CH3CH�TCH2��+NaBr+H2O | |
| C�� | 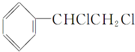 +2NaOH$��_{��}^{�Ҵ�}$ +2NaOH$��_{��}^{�Ҵ�}$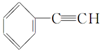 +2NaCl+2H2O +2NaCl+2H2O | |
| D�� | 2CH3OH$��_{��}^{Ũ����}$CH3-O-CH3+H2O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com