
���� ��1����A��D��ˮ��Һ����ʹ��ɫʯ����ֽ��죬����Һ���ԣ���ת����ϵ��֪�����б��Ԫ�أ�AΪH2S��BΪSO2��CΪSO3��DΪH2SO4������ת����ϵ��
��2����A��ˮ��Һ��ʹ��ɫʯ����ֽ��������ҺΪ���ԣ���ת����ϵ��֪�����б��Ԫ�أ�D��ϡ��Һ��ʹ��ɫʯ����ֽ��죬��ҺΪ���ԣ���AΪNH3��BΪNO��CΪNO2��DΪHNO3������ת����ϵ��
��� �⣺��1����A��D��ˮ��Һ����ʹ��ɫʯ����ֽ��죬����Һ���ԣ���ת����ϵ��֪�����б��Ԫ�أ�AΪH2S��BΪSO2��CΪSO3��DΪH2SO4��A��Bת���ķ���ʽ��2H2S+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2SO2+2H2O��
�ʴ�Ϊ��H2S��H2SO4��2H2S+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2SO2+2H2O��
��2����A��ˮ��Һ��ʹ��ɫʯ����ֽ��������ҺΪ���ԣ���ת����ϵ��֪�����б��Ԫ�أ�D��ϡ��Һ��ʹ��ɫʯ����ֽ��죬��ҺΪ���ԣ���AΪNH3��BΪNO��CΪNO2��DΪHNO3����������������NO��ˮ����Ӧ����ʽΪ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
�ʴ�Ϊ��NH3��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
���� ���⿼��������ƶϣ�Ϊ��Ƶ���㣬����ת���е���������������ǿ��Ϊ�ƶϵĹ�ϵ������N���仯���S���仯�������ʵĿ��飬��Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij��Һ�еμ������ܲ�����ɫ���壬��������ʹ����ʯ��ˮ����ǣ���ԭ��Һ��һ����CO32- | |
| B�� | ��ij��Һ�м���BaCl2��Һ���ְ�ɫ�����������Һ�п϶���SO42- | |
| C�� | ��ij��Һ�м���NaOH��Һ���ټ��ȣ���������������ʹʪ���ɫʯ����ֽ�����������Һ�п϶���NH4+ | |
| D�� | ����ɫ��Ӧ�ɼ����NaCl��KCl��Na2SO4�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4HCO3��Һ�����NaOH��Һ��Ӧ��NH4++OH-�TNH3��+H2O | |
| B�� | ��̼�������Һ�е���������NaOH��Һ��HCO3-+Ca2++OH-�TCaCO3��+H2O | |
| C�� | ��Ba��OH��2��Һ�м������NH4HSO4��Һ��Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O | |
| D�� | ����Ͱ�ˮ��Ӧ��HOOC-COOH+2NH3•H2O�TC2O42-+2NH4++2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ���ϴ�ͼ��Ŀ�����NaAlO2��Һ����μ������� | |
| B�� | 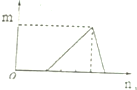 ���ϴ�ͼ��Ŀ��������������AlCl3��Һ�еμ�NaOH��Һ | |
| C�� | 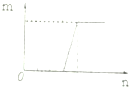 ���ϴ�ͼ��Ŀ��������е����ʵ�����NaOH��Na2CO3��Һ�еμ����� | |
| D�� | 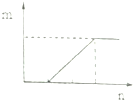 ���ϴ�ͼ������е����ʵ�����NaHCO3��Na2CO3����Һ�еμ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�Ӧ���ڷ��ȷ�Ӧ | B�� | �Ͽ�H-H���ų����� | ||
| C�� | �γ�O-H���������� | D�� | ��Ӧ������������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ar�Ľṹʾ��ͼ�� | B�� | CO2�ķ���ģ��ʾ��ͼ�� | ||
| C�� | CSO�ĵ���ʽ�� | D�� | N2�Ľṹʽ����N��N�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1��3 | B�� | 1��7 | C�� | 1��1 | D�� | 5��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
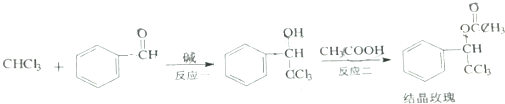
| A�� | ��Ӧһ�Ǽӳɷ�Ӧ | |
| B�� | ��Ӧ���ķ�������Ũ���������� | |
| C�� | �ᾧõ�����ʽΪC10H9Cl3O2 | |
| D�� | ���������������л���Ĺ����Ź���6�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com