
¢ÅCH3£«£®CH3££®CH3£¶¼ŹĒÖŲŅŖµÄÓŠ»ś·“Ó¦ÖŠ¼äĢ壬ӊ¹ŲĖüĆĒµÄĖµ·ØÕżČ·µÄŹĒ ”£
A£®ĖüĆĒ¾łÓɼ×ĶéČ„µōŅ»øöĒāŌ×ÓĖłµĆ
B£®ĖüĆĒ»„ĪŖµČµē×ÓĢ壬Ģ¼Ō×Ó¾ł²ÉČ”sp2ŌÓ»Æ
C£®CH3£ÓėNH3£®H3O+»„ĪŖµČµē×ÓĢ壬¼øŗĪ¹¹ŠĶ¾łĪŖČż½Ē׶ŠĪ
D£®CH3£«ÖŠµÄĢ¼Ō×Ó²ÉČ”sp2Ōӻƣ¬ĖłÓŠŌ×Ó¾ł¹²Ćę
E£®Į½øöCH3£»ņŅ»øöCH3£«ŗĶCH3£½įŗĻ¾łæɵƵ½CH3CH3
£Ø2£©ŠæŹĒŅ»ÖÖÖŲŅŖµÄ½šŹō£¬Šæ¼°Ęä»ÆŗĻĪļӊ׏ć·ŗµÄÓ¦ÓĆ”£
¢ŁÖø³öŠæŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ£ŗ ÖÜĘŚ£¬ ×壬 Ēų”£
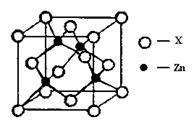
¢ŚĘĻĢŃĢĒĖįŠæ[CH2OH£ØCHOH£©4COO]2ZnŹĒÄæĒ°ŹŠ³”ÉĻĮ÷ŠŠµÄ²¹Šæ¼Į”£Š“³öZn2+»łĢ¬µē×ÓÅŲ¼Ź½ £»ĘĻĢŃĢĒ·Ö×ÓÖŠĢ¼Ō×ÓŌӻƷ½Ź½ÓŠ ”£
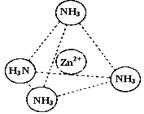
¢ŪZn2+ÄÜÓėNH3ŠĪ³ÉÅäĄė×Ó[Zn£ØNH3£©4]2+”£ÅäĪ»ĢåNH3·Ö×ÓŹōÓŚ £ØĢī”°¼«ŠŌ·Ö×Ó”±»ņ”°·Ē¼«ŠŌ·Ö×Ó”±£©£»ŌŚ[Zn£ØNH3£©4]2+ÖŠ£¬Zn2+Ī»ÓŚÕżĖÄĆęĢåÖŠŠÄ£¬NĪ»ÓŚÕżĖÄĆęĢåµÄ¶„µć£¬ŹŌŌŚ×óĻĀĶ¼ÖŠ±ķŹ¾[Zn£ØNH3£©4]2+ÖŠZn2+ÓėNÖ®¼äµÄ»Æѧ¼ü”£
 | |||
 | |||
¢ÜÓŅÉĻĶ¼±ķŹ¾ŠæÓėij·Ē½šŹōŌŖĖŲXŠĪ³ÉµÄ»ÆŗĻĪļ¾§°ū£¬ĘäÖŠZnŗĶXĶعż¹²¼Ū¼ü½įŗĻ£¬øĆ»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖ £»øĆ»ÆŗĻĪļµÄ¾§ĢåČŪµć±Čøɱłøߵƶą£¬ŌŅņŹĒ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ¶ž¼×»łŃĒķæ
¶ž¼×»łŃĒķæ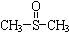 ÓŠĻūŃ×Ö¹Ķ“”¢Õņ¾²µČ×÷ÓĆ£®¼×“¼ŗĶĮņ»ÆĒāŌŚ¦Ć-Al2O3“߻ƼĮ×÷ÓĆĻĀÉś³É¼×ĮņĆŃ£ØCH3-S-CH3£©£¬¼×ĮņĆŃŌŁÓėNO2·“Ó¦ÖĘČ”¶ž¼×»łŃĒķæ£Ø
ÓŠĻūŃ×Ö¹Ķ“”¢Õņ¾²µČ×÷ÓĆ£®¼×“¼ŗĶĮņ»ÆĒāŌŚ¦Ć-Al2O3“߻ƼĮ×÷ÓĆĻĀÉś³É¼×ĮņĆŃ£ØCH3-S-CH3£©£¬¼×ĮņĆŃŌŁÓėNO2·“Ó¦ÖĘČ”¶ž¼×»łŃĒķæ£Ø £©£¬ÓŠ¹Ų·“Ó¦ČēĻĀ£ŗ
£©£¬ÓŠ¹Ų·“Ó¦ČēĻĀ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¢ÅCH3£«£®CH3££®CH3£¶¼ŹĒÖŲŅŖµÄÓŠ»ś·“Ó¦ÖŠ¼äĢ壬ӊ¹ŲĖüĆĒµÄĖµ·ØÕżČ·µÄŹĒ ”£
A£®ĖüĆĒ¾łÓɼ×ĶéČ„µōŅ»øöĒāŌ×ÓĖłµĆ
B£®ĖüĆĒ»„ĪŖµČµē×ÓĢ壬Ģ¼Ō×Ó¾ł²ÉČ”sp2ŌÓ»Æ
C£®CH3£ÓėNH3”¢H3O+»„ĪŖµČµē×ÓĢ壬¼øŗĪ¹¹ŠĶ¾łĪŖČż½Ē׶ŠĪ
D£®CH3£«ÖŠµÄĢ¼Ō×Ó²ÉČ”sp2Ōӻƣ¬ĖłÓŠŌ×Ó¾ł¹²Ćę
E£®Į½øöCH3£»ņŅ»øöCH3£«ŗĶŅ»øöCH3£½įŗĻ¾łæɵƵ½CH3CH3
£Ø2£©ŠæŹĒŅ»ÖÖÖŲŅŖµÄ½šŹō£¬Šæ¼°Ęä»ÆŗĻĪļӊ׏ć·ŗµÄÓ¦ÓĆ”£
¢ŁÖø³öŠæŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ£ŗµŚ ÖÜĘŚ£¬µŚ ×壬ŹōÓŚ Ēų”£
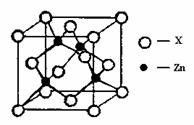
¢ŚĘĻĢŃĢĒĖįŠæ[CH2OH£ØCHOH£©4COO]2ZnŹĒÄæĒ°ŹŠ³”ÉĻĮ÷ŠŠµÄ²¹Šæ¼Į”£Š“³öZn2+»łĢ¬µē×ÓÅŲ¼Ź½ £»ĘĻĢŃĢĒ·Ö×ÓÖŠĢ¼Ō×ÓŌӻƷ½Ź½ÓŠ ”£
¢ŪZn2+ÄÜÓėNH3ŠĪ³ÉÅäĄė×Ó[Zn£ØNH3£©4]2+”£ÅäĪ»ĢåNH3·Ö×ÓŹōÓŚ £ØĢī”°¼«ŠŌ·Ö×Ó”±»ņ”°·Ē¼«ŠŌ·Ö×Ó”±£©£»ŌŚ[Zn£ØNH3£©4]2+ÖŠ£¬Zn2+Ī»ÓŚÕżĖÄĆęĢåÖŠŠÄ£¬NĪ»ÓŚÕżĖÄĆęĢåµÄ¶„µć£¬ŹŌŌŚ×óĻĀĶ¼ÖŠ±ķŹ¾³ö[Zn£ØNH3£©4]2+ÖŠZn2+ÓėNÖ®¼äµÄ»Æѧ¼ü”£
 | |||
 | |||
¢ÜÓŅÉĻĶ¼±ķŹ¾ŠæÓėij·Ē½šŹōŌŖĖŲXŠĪ³ÉµÄ»ÆŗĻĪļ¾§°ū£¬ĘäÖŠZnŗĶXĶعż¹²¼Ū¼ü½įŗĻ£¬øĆ»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖ £»øĆ»ÆŗĻĪļ¾§ĢåµÄČŪµć±Čøɱł £ØĢīŠ“”°øß”±»ņ”°µĶ”±£©£¬ŌŅņŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011ÄźÕć½Ź”ŗ¼ÖŻŹŠĻōɽĒųøßæ¼»ÆŃ§Ä£ÄāŹŌ¾ķ£ØŹ®°Ė£©£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
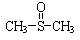 £©£¬ÓŠ¹Ų·“Ó¦ČēĻĀ£ŗ
£©£¬ÓŠ¹Ų·“Ó¦ČēĻĀ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ0113 ĘŚÄ©Ģā ĢāŠĶ£ŗµ„Ń”Ģā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com