
����Ŀ�����û�ѧԭ���Է�������ˮ������������̼��������ʵ����ɫ�������������ã��Թ�����̬��������Ҫ���塣
��������
(1)H2��ԭ��������������
��֪��N2(g)��2O2(g)=2NO2(g) ��H1=+133 kJ/mol
H2O(g)=H2O(l) ��H2=��44 kJ/mol
H2��ȼ������H3=��285.8 kJ/mol
�ڴ��������£�H2��ԭNO2����ˮ�����͵������Ȼ�ѧ����ʽΪ__________��
(2)��NH3����ԭ�������������������Ӧ��
4NH3(g)��6NO(g)![]() 5N2(g)��6H2O(l)����H<0
5N2(g)��6H2O(l)����H<0
��ͬ�����£���2L�����ܱ������У�ѡ�ò�ͬ����������N2������ʱ��仯��ͼ��ʾ��
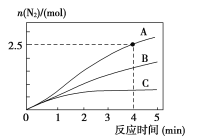
�ټ���0��4������A���������£���Ӧ����v(NO)=____��
������˵����ȷ����____��
A.�÷�Ӧ�Ļ�ܴ�С˳���ǣ�Ea(A)>Ea(B)>Ea(C)
B.����ѹǿ��ʹ��Ӧ���ʼӿ죬����Ϊ�����˻���Ӱٷ���
C.��λʱ����H��O����N��H�����ѵ���Ŀ���ʱ��˵����Ӧ�Ѵﵽƽ��
D.����Ӧ�ں��ݾ��ȵ��ܱ������н��У���Kֵ����ʱ��˵���Ѵﵽƽ��
(3)����ԭ��ط�Ӧ��ʵ��NO2���������ܷ�ӦΪ6NO2+8NH3=7N2+12H2O���������ҺΪNaOH��Һ������һ��ʱ��õ��������������ҺpH_____(��������������С������������)�������ĵ缫��ӦʽΪ________��
����̼��
(4)�ü״���CO��Ӧ���ɴ��������CO��Ⱦ�������£���a mol/L������b mol/L Ba(OH)2��Һ�������ϣ���ַ�Ӧ����Һ�д���2c(Ba2+)=c(CH3COO-)��������Һ����仯���������ĵ��볣��Ka=________(�ú�a��b�Ĵ���ʽ��ʾ)��
���𰸡�4H2(g)��2NO2(g)= N2(g)��4H2O(g) ��H=-1100.2 kJ/mol 0.375 mol/(L��min) CD ���� 2NH3-6e-+6OH-=N2+6H2O ![]() ��10-7mol/L
��10-7mol/L
��������
(1)�����Ȼ�ѧ����ʽ��˹���ɼ��������Ȼ�ѧ����ʽ��
(2)����֪��A���������£�4minʱ����Ϊ2.5mol�������ĵ�NOΪ3mol������v(NO)=![]() ���㣻
���㣻
��A.��ͬʱ�������ɵĵ��������ʵ���Խ�࣬��Ӧ����Խ�죬���Խ�ͣ�
B.�ı�ѹǿ������Ӱٷ������䣻
C.��λʱ����H-O�����ѱ�ʾ�����ʣ�N-H�����ѱ�ʾ�����ʣ�����������ͬ��Ӧ�Ѿ��ﵽƽ�⣻
D.�÷�ӦΪ���ȷ�Ӧ�����ݾ��ȵ��ܱ������У���Ӧʱ�¶Ȼ����ߣ���K���С��
(3)�������Ƕ��������õ����ӷ�����ԭ��Ӧ���������ǰ���ʧ���ӷ���������Ӧ����ϵ缫��Ӧ�����жϣ�
(4)��Һ������������Ũ�ȼ���һ�룬�������ƽ�ⳣ����Ũ���أ����K�Ķ�����㡣
�� ��֪��H2��ȼ����Ϊ285.8kJ/mol��
�� H2(g)+![]() O2(g)=H2O(l) ��H=-285.8kJ/mol��
O2(g)=H2O(l) ��H=-285.8kJ/mol��
�� N2(g)+2O2(g)=2NO2(g) ��H=+133 kJ/mol
�� H2O(g)=H2O(l) ��H=-44 kJ/mol
���ݸ�˹���ɣ�����4-��-����4���ڴ��������£�H2��ԭNO2����ˮ���������������ʵ��Ȼ�ѧ����ʽΪ4H2(g)��2NO2(g)= N2(g)��4H2O(g) ��H=-1100.2 kJ/mol��
(2)����֪��A����������4minʱ����Ϊ2.5mol�������ĵ�NOΪ3mol������v(NO)=![]() =0.375 mol/(L��min)��
=0.375 mol/(L��min)��
��A.��ͬʱ�������ɵĵ��������ʵ���Խ�࣬��Ӧ����Խ�죬���Խ�ͣ����Ը÷�Ӧ�Ļ�ܴ�С˳���ǣ�Ea(A)��Ea(B)��Ea(C)��A����
B.����ѹǿ��ʹ��Ӧ���ʼӿ죬����Ϊ�����˻������������Ӧ��Ļ���Ӱٷ������䣬B����
C.��λʱ����H-O�����ѱ�ʾ�����ʣ�N-H�����ѱ�ʾ�����ʣ���λʱ����H-O����N-H�����ѵ���Ŀ���ʱ�������ĵ�NH3�����ĵ�ˮ�����ʵ���֮��Ϊ4��6������������֮�ȵ���4��6��˵����Ӧ�Ѿ��ﵽƽ��״̬��C��ȷ��
D.�÷�ӦΪ���ȷ�Ӧ�����ݾ��ȵ��ܱ������У���Ӧʱ�¶Ȼ����ߣ����¶Ȳ���ʱ��˵����Ӧ�Ѿ��ﵽƽ�⣬D��ȷ��
�ʺ���ѡ����CD��
(3)�������ܷ�Ӧ����ʽΪ6NO2+8NH3=7N2+12H2O���������ϣ�NO2�õ����ӷ�����ԭ��Ӧ���缫��ӦΪ��2NO2+8e-+4H2O=N2+8OH-�����ݵ缫��Ӧʽ��֪���õ��������������Һc(OH-)�еģ���Һ��pH�����ڸ�����NH3ʧ���ӷ���������Ӧ���缫��ӦʽΪ��2NH3-6e-+6OH-=6H2O+N2��
(4)��Ӧƽ��ʱ��2c(Ba2+)=c(CH3COO-)=bmol/L���ݵ���غ��֪��c(H+)=c(OH-)=10-7mol/L����Һ�����ԣ���������ƽ�ⳣ��ΪK=![]() =
=![]() =
=![]() ��10-7mol/L��
��10-7mol/L��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() ��һ������Ӫ��ǿ������һ������ˮ�ܿ���Ҫ�ɷ�Ϊ
��һ������Ӫ��ǿ������һ������ˮ�ܿ���Ҫ�ɷ�Ϊ![]() ��
��![]() ����������
����������![]() ��
��![]() ��MnO�ȣ�����ȡ
��MnO�ȣ�����ȡ![]() �Ĺ���������ͼ1��
�Ĺ���������ͼ1��
��֪��������Һ���е���������Ҫ��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() �ȣ�
�ȣ�
������������������������ʽ����ʱ��Һ��pH����������������Ũ��Ϊ��0.01mol/L��
��![]() �۵�Ϊ86����������110~120��ʱ��ʧȥ�ᾧˮ������ˮ�����ܡ�
�۵�Ϊ86����������110~120��ʱ��ʧȥ�ᾧˮ������ˮ�����ܡ�
������ |
|
|
|
|
|
��ʼ���� | 2.7 | 7.6 | 7.6 | 4.0 | 7.7 |
��ȫ���� | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
��1��д������������![]() ������Ӧ�����ӷ���ʽ____��
������Ӧ�����ӷ���ʽ____��
��2��д��NaClO3������Ӧ����Ҫ���ӷ���ʽ____����������������Һ���мӹ���NaClO3ʱ�����ܻ������ж����壬д�����ɸ��ж���������ӷ���ʽ____��
��3������![]() ��pH��a�����������õ��ij����ɷ�Ϊ___��
��pH��a�����������õ��ij����ɷ�Ϊ___��
��4���Ƶõ�![]() �ں��ʱ���ѹ��ɵ�Դ����___��
�ں��ʱ���ѹ��ɵ�Դ����___��
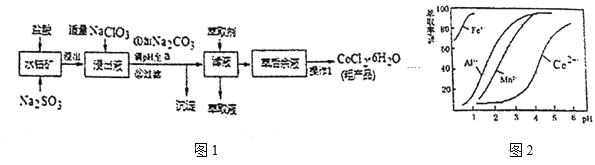
��5����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ2��������Һ���м�����ȡ����Ŀ����___�r��ʹ�õ����pH��Χ��___��
A��2.0~2.5 B��3.0~3.5 C��4.0~4.5 D��5.0~5.5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ͬѧ������ʵ��̽��Fe2+��Fe3+������,�ش��������⣺
(1)�ֱ�ȡһ�����Ȼ������Ȼ���������,�����Ƴ�0.1mol/L����Һ����FeCl2��Һ�������������м,��Ŀ���� ______ ��
(2)����ͬѧȡ2mLFeCl2��Һ,���뼸����ˮ,�ټ���1��KSCN��Һ,��Һ���,˵��Cl2�ɽ�Fe2+������FeCl2��Һ����ˮ��Ӧ�����ӷ���ʽΪ ______
(3)����ͬѧ��Ϊ�����ʵ�鲻���Ͻ�,����ͬѧ��2mLFeCl2��Һ���ȼ���0.5mLú��,����Һ�������μ��뼸����ˮ��1��KSCN��Һ,��Һ���,ú�͵������� ______ ��
(4)����ͬѧȡ10mL0.1mol/LKI��Һ,����6mL0.1mol/L FeCl3��Һ��ϡ��ֱ�ȡ2mL����Һ��3֧�Թ��н�������ʵ�飺
�ٵ�һ֧�Թ��м���1mLCCl4���������, CCl4������ɫ��
�ڵڶ�֧�Թ��м���1��K3[Fe(CN)6]��Һ,������ɫ������
�۵���֧�Թ��м���1��KSCN��Һ,��Һ��졣
ʵ��ڼ���������� ______(�����ӷ���)��ʵ��ٺ͢�˵������I-�����������,��Һ���Ժ��� ______(�����ӷ���),��Ӧ�����ӷ���ʽΪ ______ ��
(5)����ͬѧ��ʢ��H2O2��Һ���Թ��м��뼸���ữ��FeCl2��Һ,��Һ����ػ�ɫ,������Ӧ�����ӷ���ʽΪ ______ ��һ��ʱ���,��Һ�������ݳ���,������,����к��ɫ��������,�������ݵ�ԭ���� ______ ,���ɳ�����ԭ���� ______(��ƽ���ƶ�ԭ������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��ȷ����
A.Na2S2O3��Һ��ϡH2SO4��Ӧ��S2O32��+6H+=2S��+3H2O
B.�ں����ҵĽ���Һ�еμ�H2O2�õ�I2��2I+H2O2+2H+=I2+O2��+2H2O
C.�� NH4Al(SO4)2��Һ�м���Ba(OH)2��Һ��SO42��ǡ�ó�����ȫ��Al3++2SO42��+2Ba2++4OH��=AlO2��+2BaSO4��+2H2O
D.Fe��ϡ���ᷴӦ����n(Fe)��n(HNO3)=1��2ʱ��3Fe+2NO3��+8H+=3Fe2++2NO��+4H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ�� A��B��C��D��E��F ����Ԫ�أ����ǵ�ԭ�������� A �� F�������������ڱ��У�A ��ԭ�Ӱ뾶��С��B Ԫ�ص�ԭ���������������ڲ�� ������������C Ϊ�ؿ��к�������Ԫ�أ�D ��ԭ�Ӱ뾶���Ķ���������Ԫ�أ�D ����ȼ��ʱ���ֻ�ɫ���棬D �ĵ����ڸ������� C �ĵ��ʳ�ַ�Ӧ�����Եõ��� E ������ɫ��ͬ�ĵ���ɫ��̬�����D �� F �γɵĻ����� DF �dz��õĵ�ζƷ���Ը� �����������ش�
(1)Ԫ�ط��ţ�A____________��B____________��C____________��D____________��
(2)E ��Ԫ�����ڱ��е�λ�ã�_____________________��
(3)F ���ӽṹʾ��ͼ��______________________��
(4)A��B ��ɵ���������������_____��
(5)C ��D ��ԭ�Ӹ����� 1��1 ��ɵ�һ�ֻ�������ˮ������Ӧ�Ļ�ѧ����ʽΪ_____________________��
(6) ��˵�� E �ķǽ����Ա� F �ķǽ�����_____( ����ǿ����������) ����ʵ��____(��һ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Һ����(��ͨ��)������(������Ϊ�������Һ)�����ɳ����������������ҵ�������ϵ���߷���ͼ����

A.��Na[Al(OH)4]��Һ��ͨ�������̼
B.��Na[Al(OH)4]��Һ�еμ�����
C.�����ʯ��ˮ��ͨ�������̼
D.��Al2(SO4)3��Һ�еμ�NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��ش��������⣺
(1)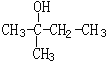 ��ϵͳ������______________________����H-NMR������________���壬��������Ϊ___________________
��ϵͳ������______________________����H-NMR������________���壬��������Ϊ___________________
(2)д�� 2��3��������4���һ�����ṹ��ʽ_________________CH3CH2CH2CHO �ļ���ʽ___________________________________________________________
(3)д��CH3CH2OH�������Ļ�ѧ����ʽ��__________________CH3CH2Br��ȡ�Ҵ��Ļ�ѧ����ʽ��______________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʣ���Ar ��Na2O2 ��AlCl3 ��HClO ��N2 ��MgF2 ��NH4Cl
(1)ֻ���ڹ��ۼ�����___�������ڻ�ѧ������_____,���ӻ�������_______
(2)�����Ӽ��ͼ��Լ����ɵ�������__________
(3)N2�ĵ���ʽΪ_____ HClO�ĵ���ʽΪ________NH4Cl�ĵ���ʽΪ___________
(4)�õ���ʽ��ʾMgF2���γɹ���___________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��b��![]() ����d��
����d��![]() ����p��
����p��![]() �ķ���ʽ��ΪC6H6������˵����ȷ����
�ķ���ʽ��ΪC6H6������˵����ȷ����
A. b��ͬ���칹��ֻ��d��p����B. b��d��p�Ķ��ȴ����ֻ������
C. b��d��p���������Ը��������Һ��ӦD. b��d��p��ֻ��b������ԭ�Ӵ���ͬһƽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com