
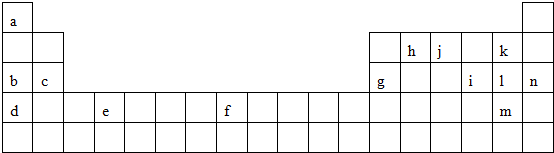

 ��
������ ��Ԫ�������ڱ���λ�ÿ�֪��a��n��Ԫ�طֱ�ΪH��Na��Mg��K��Ti��Fe��Al��C��N��F��S��Cl��Br��Ar��
��1������Ԫ���У�F�ķǽ�������ǿ��K�Ľ�������ǿ��
��2��c������������ˮ����Ϊ������þ��
��3��a��j�γɵĻ�����Ϊ������
��4����ѧ��������õ�Ԫ��ΪAr��
��5��iԪ��ΪS��λ�ڵ������ڵڢ�A��
��6��gԪ���γɵ���������b������������ˮ������Һ��Ӧ������ƫ�����ƺ�ˮ��
��7���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ���
��� �⣺��Ԫ�������ڱ���λ�ÿ�֪��a��n��Ԫ�طֱ�ΪH��Na��Mg��K��Ti��Fe��Al��C��N��F��S��Cl��Br��Ar��
��1������ЩԪ���У�����õķǽ���Ԫ����F������õĽ���Ԫ����K���ʴ�Ϊ��F��K��
��2��c������������ˮ����Ļ�ѧʽΪMg��OH��2���ʴ�Ϊ��Mg��OH��2��
��3��a��j�γɵĻ�����Ϊ�����������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4����ѧ��������õ�Ԫ��ΪAr��ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��5��iԪ�������ڱ��е�λ���ǵ������� �ڢ�A���ʴ�Ϊ���������� �ڢ�A��
��6��gԪ���γɵ���������b������������ˮ������Һ��Ӧ�����ӷ���ʽΪAl2O3+2OH-?=2AlO2-+H2O������Al2O3+2OH-+3H2O?=2[Al��OH��4]- ����
�ʴ�Ϊ��Al2O3+2OH-?=2AlO2-+H2O����Al2O3+2OH-+3H2O?=2[Al��OH��4]- ����
��7���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ�����h��j��k��Ԫ�ص�����⻯����ȶ�����ǿ�������е�˳��ΪHF��NH3��CH4 ���ʴ�Ϊ��HF��NH3��CH4 ��
���� ���⿼��λ�á��ṹ�����ʣ�Ϊ��Ƶ���㣬����Ԫ�ص�λ�á����ʡ�Ԫ�������ɼ�Ԫ�ػ�����֪ʶΪ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע�⻯ѧ�����Ӧ�ã���Ŀ�ѶȲ���


 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | NO2 | B�� | NO | C�� | NH3 | D�� | N2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
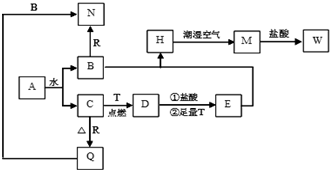
 �ļ�������麟������������Ƭ���ù������ʹ��̼�����ҵ����ʯīΪ�缫����ļ��Ȼ����Һ�Ʊ��ļ�������� ��Һ��ԭ����ͼ��ʾ������˵������ȷ���ǣ�������
�ļ�������麟������������Ƭ���ù������ʹ��̼�����ҵ����ʯīΪ�缫����ļ��Ȼ����Һ�Ʊ��ļ�������� ��Һ��ԭ����ͼ��ʾ������˵������ȷ���ǣ�������| A�� | b��Ϊ������������ԭ��Ӧ | |
| B�� | a��������������ʹʪ��ĵ��۵⻯����ֽ���� | |
| C�� | ���·���ӵ�����ΪM��a��b��N | |
| D�� | ��ⷴӦ���ܻ�ѧ����ʽΪ2��CH3��4NCl+2H2O$\frac{\underline{\;���\;}}{\;}$2��CH3��4NOH+H2��+Cl2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ѹǿ���ٸı� | |
| B�� | ��λʱ��������nmolA��ͬʱ������2nmol��B | |
| C�� | C������������B���������ʵ����� | |
| D�� | ���������ܶȲ��ٸı� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H+��Na+��Cl- | B�� | H+��Ag+��Cl- | C�� | Na+��H+��NO3- | D�� | Fe2+��H+��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
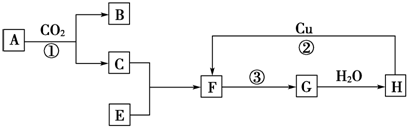
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������ˮ��2K+2H2O�T2K++OH-+H2�� | |
| B�� | С�մ���Һ�м�������NaOH��Һ��H++OH-�TH2O | |
| C�� | FeCl3��Һ��ͭ��Ӧ��Fe3++Cu�TFe2++Cu2+ | |
| D�� | ������Һ�м�������������Һ�����ԣ�Ba2++OH-+H++SO42-�TBaSO4��+H2O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com