
| A��ʵ����з�����Ӧ�����ӷ���ʽΪ��Fe2++Cl2=Fe3++2Cl-��Fe3++3SCN-=Fe��SCN��3 |
| B��ֻ��ʵ��٢ۢܣ�Ҳ�ܴﵽʵ��Ŀ�� |
| C��ͨ������ʵ���ȷ���û����ﻯѧʽΪ����NH4��2Fe��SO4��2?6H2O����һ��dz��ɫ���壬��Ʒ��ΪĦ���� |
| D��Ϊ�˼���SO42-�����Խ����е��Լ���ΪHNO3�ữ��Ba��NO3��2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��BrCH2CH2OH |
| B��BrCH2CHO |
| C��HOCH2CH2OH |
| D��BrCH2COOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
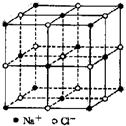 ʳ�ξ�����ͼ��ʾ���ھ����У�?��ʾNa+���� ��ʾCl-����֪ʳ�ε��ܶ�Ϊ��g/cm3��NaClĦ������Mg/mol�������ӵ�����ΪN������ʳ�ξ�����Na+��Cl-�ļ���Լ�ǣ�������
ʳ�ξ�����ͼ��ʾ���ھ����У�?��ʾNa+���� ��ʾCl-����֪ʳ�ε��ܶ�Ϊ��g/cm3��NaClĦ������Mg/mol�������ӵ�����ΪN������ʳ�ξ�����Na+��Cl-�ļ���Լ�ǣ�������A��
| |||||
B��
| |||||
C��
| |||||
D��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
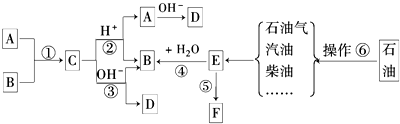
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ҺX���ȵμ�ϡ���ᣬ�ٵμ�Ba��NO3��2��Һ�����ְ�ɫ����˵����ҺX��һ������SO42- |
| B��ȡ������ҺX�������м�������������ˮ���ټӼ���KSCN��Һ����Һ��죬˵��X��Һ��һ������Fe2+ |
| C����������Ũ��Ϊ2mol?L-1��NaCl��Һ���ɽ�58.5g NaCl���뵽ʢ��500mLˮ���ձ��У����衢�ܽ� |
| D����ij���������ɫ��Ӧʵ�飬����ʻ�ɫ��˵��������Ϊһ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���þƾ���ȡ��ˮ�е��嵥�ʵIJ�����ѡ�÷�Һ©���������÷�Һ |
| B����Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| C����ȡ����Һǰ��Է�Һ©����© |
| D��Ϊ��֤��Һ©���ڵ�Һ��˳���������轫������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com