
���ܱ������н������з�Ӧ��
CO2(g)��C(s) 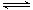 2CO(g)����H>0����ʼͨ��2molCO���ﵽƽ��ı�������������
2CO(g)����H>0����ʼͨ��2molCO���ﵽƽ��ı�������������
ָ�����ʵ�Ũ�ȡ��ٷֺ���������䡱��������С������ƽ����α仯������ơ������ơ������ƶ�������
(1)����C��ƽ��________��c(CO)________��
(2)��С�ܱ�����������������¶Ȳ��䣬��ƽ��______________��c(CO2)___ _____��
(3)ͨ��N2�������ܱ�������������¶Ȳ��䣬��ƽ��________��c(CO2)________��
(4)�����ܱ�������������䣬�����¶ȣ���ƽ��__________________��c(CO)________
��5����ѹͨ��N2��CO2�İٷֺ���
��6����ѹ��ͨ��2molCO��CO2�İٷֺ���
(10��)��(1)���ƶ������䡡(2)���淴Ӧ�����ƶ�������(3)���ƶ������䡡(4)������Ӧ�����ƶ�������5�����٣�6������
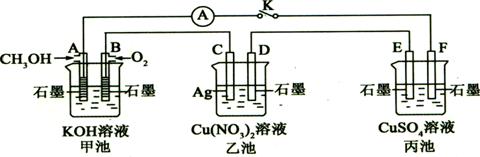
29����10�֣�ij��ȤС���ͬѧ����ͼ��ʾװ���о��йص绯ѧ������(�ס��ҡ�����������������)�����պϸ�װ�õĵ��Kʱ���۲쵽�����Ƶ�ָ�뷢����ƫת��
��ش��������⣺
��1���׳�Ϊ (�ԭ��ء��������ء���Ƴء�)��A�缫�ĵ缫��ӦʽΪ ��
��2��������F�缫Ϊ (�����������������������������������)���óص��ܷ�Ӧ��ѧ����ʽΪ ��
��3�����ҳ���C����������10.8 gʱ���׳���B�缫����������O2�����Ϊ mL(��״��)��
��4��һ��ʱ��Ͽ����K������������ʹ�ҳػָ�����ӦǰŨ�ȵ��� (��ѡ����ĸ)��
A��Cu B��CuO C��Cu(OH)2 D��Cu2(OH)2CO3
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�ܱ������з���ס��ҡ��������������ʣ�һ��ʱ������������£�
| �������� | �� | �� | �� | �� |
| ��Ӧǰ����(g) | 25 | 15 | 1 | 5 |
| ��Ӧ������(g) | 11 | δ�� | 1 | 22 |
�����б�����ȷ����
A�� δ��ֵΪ3 g B����һ���Ǵ���
C.��ȫ���μӷ�Ӧ D�������ҷ�Ӧ��������Ϊ14��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
6 g Na2X��Na+�����ʵ���Ϊ0.4 mol����Na2X��Ħ������Ϊ________��X�����ԭ������Ϊ________������������Ʒ�ܽ���ˮ�����200 mL����Һ����������Һ�����ʵ���Ũ��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�٢ڢۢ� ���ֽ���Ƭ������������ϡ�����ж������ԭ��� ���٢�����ʱ�����·�����Ӣ������ ���٢�����ʱ����Ϊ����;�ڢ�����ʱ�����������ݳ� ���� �� ����ʱ���� ���������� ���ݴ��ж������ֽ�������ɴ�С��˳���ǣ� ��
A . �٢ۢڢ� B . �٢ۢܢ�
C. �� �� �ڢ� D . �� �� �ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ʵ��������������ԭ�����͵��ǣ� ��
A������ɫNO2��ѹ����ɫ�ȱ�����dz
B��ʵ�����г����ű���ʳ��ˮ�ķ����ռ�������
C��SO2��������SO3�ķ�Ӧ��ʹ�ù����Ŀ�������߶��������������
D��ѹ��H2��I2��g����Ӧ��ƽ�������壬��ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ���ǣ�������
A����״���£�11.2 L NH3����1 Lˮ����Һ�к���Nԭ�ӵ�������Ϊ0.6NA
B��1.00 mol NaCl�У�����Na����������������ԼΪ8��6.02��1023
C��������1.00 L 1.00 mol��L��1��NaCl��Һ���ɽ�58.5 g NaCl����1.00 Lˮ��
D��1mol FeCl3��ȫת��ΪFe��OH��3������γ�NA��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2.0mol/LCuSO4��Һ��1.0mol/LH2SO4��Һ�������ϣ������Ϻ���Һ�������������Һ�����֮�ͣ���������Һ�м������������ۣ����㹻����ʱ���������ʣ�ࡣ��ʱ����Һ�е�Fe2+���ʵ���Ũ��Ϊ �� ��
A 0.5 mol/L B 1.5 mol/L C 1.0 mol/L D 2mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ij�л��� 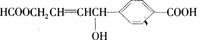 ������������ȷ���� ��������
������������ȷ���� ��������
A��1 mol���л��������3 mol Na������Ӧ��
B��1 mol���л��������3 mol NaOH������Ӧ
C��1 mol���л��������6 mol H2�����ӳɷ�Ӧ
D��1 mol���л���ֱ�������Na��NaHCO3��Ӧ����������������ͬ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����NH4�� ��Cl����Mg2����Ba2����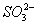 ��
��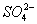 ����ȡ����20130 mL��Һ��������ʵ�飺
����ȡ����20130 mL��Һ��������ʵ�飺
(1)��һ�ݼ���AgNO3��Һ�г�������
(2)�ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.04 mol
(3)�����ݼ�����BaCl2��Һ�ø������6.27 g������������ϴ�ӡ������������Ϊ2.33 g����������ʵ�飬�����Ʋ���ȷ���� �� ��
A��K��һ������ B��20130 mL��Һ�к�0.01 mol CO32 ��
C��Cl�����ܴ��� D��Ba2��һ�������ڣ�Mg2�����ܴ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com