
����Һ�������25�棬�й�������ȷ����
| A��ij���ʵ���ҺpH<7���������һ�������ǿ�������� |
| B��pH=4��5�ķ���֭��c(H������pH=6��5��ţ����c��H��)��102�� |
| C������ʱ��ij��Һ����ˮ���������c(H��)��c(OH��)�ij˻�Ϊ1��10��24������Һ��һ�����Դ�������K����Na����AlO2����SO42�� |
| D������ʱ��0��l mol/L HA��Һ��pH>l��0��1 mol/L BOH��Һ��c(OH��)/c(H��)=l012������������Һ�������ϣ���Ϻ���Һ������Ũ�ȵĴ�С��ϵΪ��c��B����>c��OH����>c��H����>c��A���� |
B
�������������ij���ʵ���ҺpH<7��������ʳ��������ǿ�������Σ����п�������ʽ�Σ�Aѡ�����pH=4.5�ķ���֭��c(H+)��10��4.5��pH=6.5��ţ����c(H+)��10��6.5��10��4.5��10��6.5��100��Bѡ����ȷ������ʱ��ij��Һ����ˮ���������c(H��)��c(OH��)�ij˻�Ϊ1��10��24����c(H��)��c(OH��)��1��10��12����������������Һ��Ҳ�����Ǽ�����Һ��AlO2�����ڼ�����Һ���ܴ���������Һ������Ӧ��˵����Һ�п��ܴ�������K����Na����AlO2����SO42��������˵һ����Cѡ�����0.1mol��L��1 HA��Һ��pH��1��˵��HA�����ᣬ0.1mol��L��1 BOH��Һ��c��OH����/c��H����=1012����������ˮ�����ӻ����������c��OH������0.1mol��L��1�����Լ���ǿ���Ӧ����Һ���ɵ���ABˮ���Լ��ԣ�����ˮ��̶�һ�㶼�Ƚ�С����������Ũ�ȵĴ�С˳��Ϊc��B������c��A������c��OH������c��H������Dѡ�����
���㣺�����������Һ������Ũ�ȵıȽϡ�ˮ�����ӻ�������Ӧ�õȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
25��ʱ��ȡŨ�Ⱦ�Ϊ0.1 mol/L�Ĵ�����Һ�Ͱ�ˮ��Һ��20 mL���ֱ���0.1 mol/LNaOH��Һ��0.1 mol/L��������к͵ζ����ζ�������pH��μ���Һ������仯��ϵ��ͼ��ʾ������˵����ȷ����( )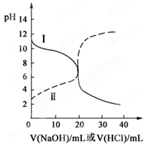
| A�����ߢμ���Һ��10 mLʱ��c(CH3COO��)��c(Na��)��c(H��)��c(OH��) |
| B�����ߢμ���Һ��20 mLʱ�� c(Cl��)��c(NH4��)��c(OH��)��c(H��) |
| C�����ߢμ���Һ��10 mL��20 mL֮����ڣ�c(NH4��)��c(Cl��)��c(OH��)��c(H��) |
| D�����ߢμ���Һ��10 mLʱ��c(CH3COO��)��c(CH3COOH)��2[c(H��)��c(OH��)] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����£���ʹpH=3��������pH=9��Ba(0H)2��Һ���ʹ���ΪpH=7����Һ�����ʱ����Һ�������Ϊ( )��
| A��1��60 | B��3��1 | C��100��l | D��1��100 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���������ε�ϡ��Һ���ֱ���a mol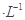 NaX��Һ��b mol
NaX��Һ��b mol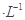 NaY��Һ������˵������ȷ����( )��
NaY��Һ������˵������ȷ����( )��
| A����a=b��pH(NaX)>pH (NaY)������ͬŨ��ʱ������HX>HY |
| B����a=b�������c��X����="c" (Y��)+c(HY)������ͬŨ��ʱ������HX>HY |
| C����a>b�����c(X��)=c(Y��)������Ƴ���Һ��c(HX)>c(HY)������ͬŨ��ʱ�� ����HX<HY |
D��������Һ�������ϣ����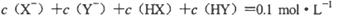 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��pH������9��NaOH��CH3COONa������Һ�У�����ˮ���������OH������Ũ�ȷֱ�ΪA mol��L��1��B mol��L��1����A��B�Ĺ�ϵΪ
| A��A��B | B��A��10��4B | C��B��10��4A | D��A��B |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
һ���¶��£�ˮ����H2O H����OH������H��Q(Q��0)��ƽ�⣬��������һ����ȷ����(����)
H����OH������H��Q(Q��0)��ƽ�⣬��������һ����ȷ����(����)
| A����ˮ�е�������ϡ���ᣬƽ�������ƶ���Kw��С |
| B����ˮ���ȣ�Kw����pH��С |
| C����ˮ�м�����������CH3COONa��ƽ�������ƶ���c(H��)���� |
| D����ˮ�м����������������ƣ�c(H��)��10��7 mol��L��1��Kw���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����������ȷ����(����)
A��Ũ�Ⱦ�Ϊ0.1 mol��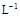 ��CH3COOH��CH3COONa��Һ�����������õ���Һ��: ��CH3COOH��CH3COONa��Һ�����������õ���Һ��:c(CH3COOH)+c(CH3COO-)="0.2" mol�� 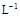 |
B��0.1 mol��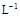 NaHCO3��Һ��:c(Na+)=c(HC NaHCO3��Һ��:c(Na+)=c(HC )+c(H2CO3)+2c(C )+c(H2CO3)+2c(C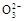 ) ) |
C��0.2 mol��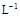 HCl��Һ��������0.1 mol�� HCl��Һ��������0.1 mol��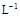 NaOH��Һ��Ϻ�,��Һ��pH=1 NaOH��Һ��Ϻ�,��Һ��pH=1 |
D��0.1 mol��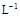 ��ˮ�е���0.1 mol�� ��ˮ�е���0.1 mol��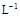 ��������Һ������ʱ,�����Һ��:c(N ��������Һ������ʱ,�����Һ��:c(N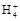 )=c(Cl-) )=c(Cl-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
0.1mol��L��1HF��Һ��pH��2�������Һ���й�Ũ�ȹ�ϵʽ����ȷ���ǣ� ��
| A��c(H��)��c(F��) | B��c(H��)��c(HF) |
| C��c(OH��)��c(HF) | D��c(HF)��c(F��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ӷ���ʽ�У�����ˮ�ⷴӦ����( )
A��HCOOH+H2O HCOO-+H3O+ HCOO-+H3O+ |
B��CO2+H2O HCO3-+H+ HCO3-+H+ |
C��CO32-+H2O HCO3-+OH- HCO3-+OH- |
D��HS-+H2O S2-+H3O+ S2-+H3O+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com