������n��CO
2��=
=0.8mol��m��C��=0.8mol��12g/mol=9.6g��
n��H
2O��=
=0.4mol��m��H��=0.4mol��2��1g/mol=0.8g
��12g���A��m��O��=12g-9.6g-0.8g=1.6g��n��O��=
=0.1mol
0.1molA�к�C��0.8mol��H��0.8mol��O��0.1mol����A�ķ���ʽΪC
8H
8O��A���Է���������Ӧ����˴Ź������������ַ壮��AΪ

��
����Ϣ���֪��

��HCN�����ӳɷ�Ӧ����B����BΪ
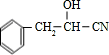
��
Bˮ������C����CΪ
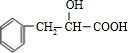
��
C��Ũ���ᡢ��������������D��D��ʹ������Ȼ�̼��Һ��ɫ��˵��C������ȥ��Ӧ����DΪ
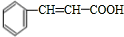
��
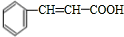
��������E��F������Ϣ���֪��������Ϊ
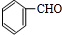
��OHC-COOH��F��������������G��G����Է�������Ϊ90����FΪOHC-COOH��EΪ

��GΪHOOC-COOH���ݴ˽��
����⣺n��CO
2��=
=0.8mol��m��C��=0.8mol��12g/mol=9.6g��
n��H
2O��=
=0.4mol��m��H��=0.4mol��2��1g/mol=0.8g
��12g���A��m��O��=12g-9.6g-0.8g=1.6g��n��O��=
=0.1mol
0.1molA�к�C��0.8mol��H��0.8mol��O��0.1mol����A�ķ���ʽΪC
8H
8O��A���Է���������Ӧ����˴Ź������������ַ壮��AΪ

��
����Ϣ���֪��

��HCN�����ӳɷ�Ӧ����B����BΪ
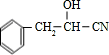
��
Bˮ������C����CΪ
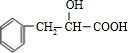
��
C��Ũ���ᡢ��������������D��D��ʹ������Ȼ�̼��Һ��ɫ��˵��C������ȥ��Ӧ����DΪ
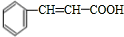
��
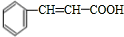
��������E��F������Ϣ���֪��������Ϊ

��OHC-COOH��F��������������G��G����Է�������Ϊ90����FΪOHC-COOH��EΪ

��GΪHOOC-COOH���ݴ˽��
��1�������Ϸ�����֪AΪ

��
�ʴ�Ϊ��

��
��2��CΪ
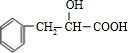
���к��������ŵ������ǻ����Ȼ���
DΪ
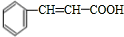
����̼̼˫����������һ�������·����Ӿ۷�Ӧ�������Ȼ�������CH
3OH����ȡ����Ӧ��������Ag��NH
3��
2OH��Һ����������Ӧ�����ܷ�����ȥ��Ӧ��
�ʴ�Ϊ���ǻ����Ȼ����٢ܣ�
��3����GΪHOOC-COOH�������Ҷ�����Ũ����������������ɣ�C
4H
4O
4��
n���ʷ��������۷�Ӧ��
��Ӧ����ʽΪ

��
�ʴ�Ϊ��

�����ۣ�
��CΪ
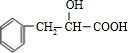
��DΪ
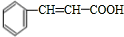
һ�������£�Cת��ΪD�ķ�ӦΪ��ȥ��Ӧ����Ӧ����ʽΪ

��
�ʴ�Ϊ��

����ȥ��
��4����Ҫ��֪��дͬ���칹��Ӧ�����Ȼ����ǻ����������з��ǻ��������ϵ�һ�ȴ���ֻ�����֣�����Ӧ������ȡ���������ڶ�λ�ϣ�Ϊ

��
�ʴ�Ϊ��4��

��










 ��
�� ��HCN�����ӳɷ�Ӧ����B����BΪ
��HCN�����ӳɷ�Ӧ����B����BΪ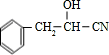 ��
��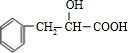 ��
��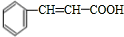 ��
��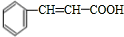 ��������E��F������Ϣ���֪��������Ϊ
��������E��F������Ϣ���֪��������Ϊ ��OHC-COOH��F��������������G��G����Է�������Ϊ90����FΪOHC-COOH��EΪ
��OHC-COOH��F��������������G��G����Է�������Ϊ90����FΪOHC-COOH��EΪ ��GΪHOOC-COOH���ݴ˽��
��GΪHOOC-COOH���ݴ˽�� ��
�� ��HCN�����ӳɷ�Ӧ����B����BΪ
��HCN�����ӳɷ�Ӧ����B����BΪ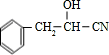 ��
��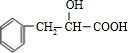 ��
��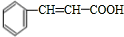 ��
��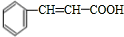 ��������E��F������Ϣ���֪��������Ϊ
��������E��F������Ϣ���֪��������Ϊ ��OHC-COOH��F��������������G��G����Է�������Ϊ90����FΪOHC-COOH��EΪ
��OHC-COOH��F��������������G��G����Է�������Ϊ90����FΪOHC-COOH��EΪ ��GΪHOOC-COOH���ݴ˽��
��GΪHOOC-COOH���ݴ˽�� ��
�� ��
��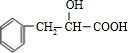 ���к��������ŵ������ǻ����Ȼ���
���к��������ŵ������ǻ����Ȼ���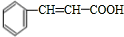 ����̼̼˫����������һ�������·����Ӿ۷�Ӧ�������Ȼ�������CH3OH����ȡ����Ӧ��������Ag��NH3��2OH��Һ����������Ӧ�����ܷ�����ȥ��Ӧ��
����̼̼˫����������һ�������·����Ӿ۷�Ӧ�������Ȼ�������CH3OH����ȡ����Ӧ��������Ag��NH3��2OH��Һ����������Ӧ�����ܷ�����ȥ��Ӧ�� ��
�� �����ۣ�
�����ۣ�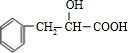 ��DΪ
��DΪ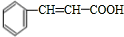 һ�������£�Cת��ΪD�ķ�ӦΪ��ȥ��Ӧ����Ӧ����ʽΪ
һ�������£�Cת��ΪD�ķ�ӦΪ��ȥ��Ӧ����Ӧ����ʽΪ ��
�� ����ȥ��
����ȥ�� ��
�� ��
��

 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
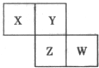 ��2012?��ɽ��ģ��X��Y��Z��W��Ϊ������Ԫ�أ����������ڱ������λ����ͼ��ʾ����Yԭ�ӵ��������������ڲ��������3��������˵������ȷ���ǣ�������
��2012?��ɽ��ģ��X��Y��Z��W��Ϊ������Ԫ�أ����������ڱ������λ����ͼ��ʾ����Yԭ�ӵ��������������ڲ��������3��������˵������ȷ���ǣ�������