




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
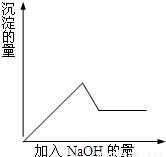
��10�֣���һ��ɫ��Һ�����п��ܺ���Fe3+.Al3+.Fe2+.Mg2+.Cu2+.K+.NO3-.SO42-�����ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��
�ڢڸ�ʵ���м���Ba(NO3)2��Һ���ɰ�ɫ���� �ڢ۸�ʵ���У����ɰ�ɫ��������
�����NaOH��������ͼ��ʾ�����ϵ���ݴ˿�֪��
����ԭ��Һ��һ�������ڵ�������_____________________��
��Ϊ�������Һ��һ�����ڵ����ӵ�Ҫ��һ����ܽ���
�ֳ�������Ϊ��д��ѧʽ��___________��_________��
��д���ڢ۸�ʵ���з�����Ӧ�����ӷ���ʽ
��
��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ��Ϫ�ظ�����ѧ��10���¿���ѧ���� ���ͣ������
��10�֣���һ��ɫ��Һ�����п��ܺ���Fe3+.Al3+.Fe2+.Mg2+.Cu2+.K+.NO3-.SO42-�����ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��

�ڢڸ�ʵ���м���Ba(NO3)2��Һ���ɰ�ɫ���� �ڢ۸�ʵ���У����ɰ�ɫ��������
�����NaOH��������ͼ ��ʾ�����ϵ���ݴ˿�֪��
��ʾ�����ϵ���ݴ˿�֪��
����ԭ��Һ��һ�������ڵ�������_____________________��
��Ϊ�������Һ��һ�����ڵ����ӵ�Ҫ��һ����ܽ���
�ֳ�������Ϊ��д��ѧʽ��___________��_________��
��д���ڢ۸�ʵ���з�����Ӧ�����ӷ���ʽ
��
��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ĩ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��ɫ��Һ�����п��ܺ���Fe3+��Al3+��Fe2+��Mg2+��Cu2+��NH4+��K+��CO32-��SO42-�������еļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��

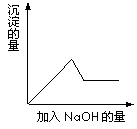 �ڢ۸�ʵ���У����ɰ�ɫ�������������NaOH��������ͼ
�ڢ۸�ʵ���У����ɰ�ɫ�������������NaOH��������ͼ
��ʾ�����ϵ���ݴ˿�֪��
����ԭ��Һ��һ�������ڵ�������____________________��
��Ϊ�������Һ��һ�����ڵ����ӵ�Ҫ��һ����ܽ���
�ֳ������ʣ��仯ѧʽ�ֱ�Ϊ___________��_________��
��д���ڢ۸�ʵ���п��ܷ��������з�Ӧ�����ӷ���ʽ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��ɫ��Һ�����п��ܺ���Fe3+.Al3+.Fe2+.Mg2+.Cu2+.K+.NO3-.SO42-�����ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��

�ڢڸ�ʵ���м���Ba(NO3)2��Һ���ɰ�ɫ���� �ڢ۸�ʵ���У����ɰ�ɫ��������
�����NaOH��������ͼ ��ʾ�����ϵ���ݴ˿�֪��
��ʾ�����ϵ���ݴ˿�֪��
����ԭ��Һ��һ�������ڵ�������_____________________��
��Ϊ�������Һ��һ�����ڵ����ӵ�Ҫ��һ����ܽ���
�ֳ�������Ϊ��д��ѧʽ��___________��_________��
��д���ڢ۸�ʵ���з�����Ӧ�����ӷ���ʽ
��
��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com