
(10��)����ЧӦ����Դ��ȱ ���������ν��ʹ����е�CO2���������Կ������������˸������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)��3H2(g)?
���������ν��ʹ����е�CO2���������Կ������������˸������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)��3H2(g)?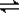 ?CH3OH(g)��H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��
?CH3OH(g)��H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��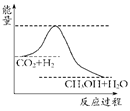
(1)���ڸ÷�Ӧ������˵���У���ȷ����________��
A����H>0����S>0
B����H>0����S<0
C����H<0����S<0
D����H<0����S>0
(2)�÷�Ӧƽ�ⳣ��K�ı���ʽΪ________________________________��
(3)�¶Ƚ��ͣ�ƽ�ⳣ��K________(����������䡱��С��)��
(4)Ϊ̽����Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(H2)________mol��L��1��min��1��
(5)���д�ʩ����ʹn(CH3OH)/n(CO2)�������________��
A�������¶�
B���������
C����H2O(g)����ϵ�з���
D���ٳ���1 mol CO2��3 mol H2
E������He(g)��ʹ��ϵ��ѹǿ����
 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д� Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g��
������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g�� CH3OH��g��+H2O��g����������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ?mol-1���ı仯��
CH3OH��g��+H2O��g����������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ?mol-1���ı仯��
| ��� | NaOH��Һ�����/mL | ��������/mL | ��Һ��pH |
| �� | 20.00 | 0.00 | 8 |
| �� | 20.00 | 20.00 | 6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)����ЧӦ����Դ��ȱ���������ν��ʹ����е�CO2���������Կ������������˸������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)��3H2(g)??CH3OH(g)��H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��
(1)���ڸ÷�Ӧ������˵���У���ȷ����________��
A����H>0����S>0
B����H>0����S<0
C����H<0����S<0
D����H<0����S>0
(2)�÷�Ӧƽ�ⳣ��K�ı���ʽΪ________________________________��
(3)�¶Ƚ��ͣ�ƽ�ⳣ��K________(����������䡱��С��)��
(4)Ϊ̽����Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(H2)________mol��L��1��min��1��
(5)���д�ʩ����ʹn(CH3OH)/n(CO2)�������________��
A�������¶�
B���������
C����H2O(g)����ϵ�з���
D���ٳ���1 mol CO2��3 mol H2
E������He(g)��ʹ��ϵ��ѹǿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������Э����߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(10��)����ЧӦ����Դ��ȱ���������ν��ʹ����е�CO2���������Կ������������˸������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)��3H2(g)? ?CH3OH(g)��H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��
?CH3OH(g)��H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��
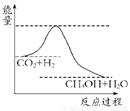
(1)���ڸ÷�Ӧ������˵���У���ȷ����________��
A����H>0����S>0
B����H>0����S<0
C����H<0����S<0
D����H<0����S>0
(2)�÷�Ӧƽ�ⳣ��K�ı���ʽΪ________________________________��
(3)�¶Ƚ��ͣ�ƽ�ⳣ��K________(����������䡱��С��)��
(4)Ϊ̽����Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(H2)________mol��L��1��min��1��

(5)���д�ʩ����ʹn(CH3OH)/n(CO2)�������________��
A�������¶�
B���������
C����H2O(g)����ϵ�з���
D���ٳ���1 mol CO2��3 mol H2
E������He(g)��ʹ��ϵ��ѹǿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�����ص���ѧ�߶���ѧ������������ѧ�Ծ� ���ͣ������
(14��)�������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӡ�
��1��Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���
Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1 L�� �ܱ������У�����1 mol CO2��3 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g)
�ܱ������У�����1 mol CO2��3 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g) ��H����49.0kJ/mol
CH3OH(g)��H2O(g) ��H����49.0kJ/mol
���CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ���ʦ�(H2)��__________mol/(L��mi n)��
n)��
�ڸ÷�Ӧ��ƽ�ⳣ������ʽΪK=__________��
��2����֪�ڳ��³�ѹ�£�
��2CH3OH(l)��3O2(g)��2CO2(g)��4H2O(g) ��H����1277 kJ��mol-1
��H����1277 kJ��mol-1
��2CO(g)��O2(g)��2CO2(g)�� ��H����566 kJ��mol-1
��C(s)+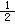 O2(g)��CO(g)�� ��H����110.5 kJ��mol-1
O2(g)��CO(g)�� ��H����110.5 kJ��mol-1
��H2O(g)��H2O(l)�� ��H����44 kJ��mol-1
��CH3OH(l)��O2(g)��CO(g)��2H2O(l)����H��________kJ��mol-1��
��3��2009��10�� ���й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��ѡ��״�ȼ�ϵ�صĹ���ԭ������ͼ��ʾ��
���й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��ѡ��״�ȼ�ϵ�صĹ���ԭ������ͼ��ʾ��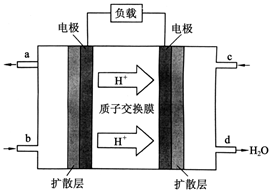
�ٸõ�ع���ʱ��b��ͨ�������Ϊ___________��c��ͨ�������Ϊ___________��
�ڸõ�ظ����ĵ缫��ӦʽΪ��_________________��
�۹���һ��ʱ������·����1.2NA������ͨ��ʱ���� g�״��μӷ�Ӧ��
��4��������ѡ������״���Ϊͬϵ�����_______(����ĸ����)����ͬϵ������Է���������ͬ�����ᷴӦ�Ļ�ѧ����ʽΪ______________���÷�Ӧ�ķ�Ӧ������____________��
��д��һ����Dѡ�Ϊͬ���칹���Һ��б���������_______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com