
ĻņČŻ»żĪŖ2 LµÄĆܱÕČŻĘ÷ÖŠ³äČĖ2 mol AĘųĢåŗĶ1 mol BĘųĢ壬ŌŚŅ»¶ØĢõ¼žĻĀ·¢ÉśČēĻĀ·“Ó¦£ŗ2A(g)£«B(g)  3C(g)£»¾2 sŗó“ļµ½Ę½ŗā£¬²āµĆCĘųĢåµÄÅضČĪŖ0.6
mol”¤L£1”£ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ
3C(g)£»¾2 sŗó“ļµ½Ę½ŗā£¬²āµĆCĘųĢåµÄÅضČĪŖ0.6
mol”¤L£1”£ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ
¢ŁÓĆĪļÖŹA±ķŹ¾øĆ·“Ó¦µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ0.2 mol”¤L£1”¤s£1
¢ŚÓĆĪļÖŹB±ķŹ¾øĆ·“Ó¦µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ0.2 mol”¤L£1”¤s£1
¢ŪĘ½ŗāŹ±ĪļÖŹAÓėBµÄ×Ŗ»ÆĀŹĻąµČ
¢ÜĘ½ŗāŹ±ĪļÖŹBµÄÅضČĪŖ0.2 mol”¤L£1
¢ŻĘäĖūĢõ¼ž²»±ä£¬ĻņČŻĘ÷ÖŠŌŁ¼ÓČė1 molCĘųĢ壬“ļµ½ŠĀĘ½ŗāŹ±£¬CµÄĢå»ż·ÖŹż²»±ä
A£®¢Ł¢Ū¢Ż””””””””B£®¢Ł¢Ś¢Ū””””””””C£®¢Ś¢Ü¢Ż””””””””D£®¢Ł¢Ū¢Ü
 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø1£©Čō±£³ÖŃ¹Ē攢ĪĀ¶Č²»±ä£¬ĻņøĆČŻĘ÷ĄļŌŁĶØČė1 mol SO2ŗĶ0.5 mol O2£¬ŌņĘ½ŗāŹ±SO3µÄŗ¬Įæ___________A%£ØĢī”°“óÓŚ”±”°µČÓŚ”±»ņ”°Š”ÓŚ”±£¬ĻĀĶ¬£©”£
£Ø2£©Čō±£³ÖŃ¹Ē攢ĪĀ¶Č²»±ä£¬ĻņøĆČŻĘ÷ĄļĶØČė1 mol N2,ŌņĘ½ŗāŹ±SO3µÄŗ¬Įæ________A%”£
£Ø3£©Čō±£³ÖĢå»ż”¢ĪĀ¶Č²»±ä£¬ĻņøĆČŻĘ÷ÖŠĶØČė1 mol N2,ŌņĘ½ŗāŹ±SO3µÄŗ¬Įæ________A%”£
£Ø4£©Čō±£³ÖĢå»ż²»±ä£¬ĪĀ¶ČÉżøß100 ”ę£¬“ĖĪĀ¶ČĻĀČŻĘ÷ÄŚµÄŃ¹Ēæ____p£¬Ę½ŗāĻņ_____·½ĻņŅĘ¶Æ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»¶ØĪĀ¶ČĻĀ£¬ĻņČŻ»żĪŖ2 LµÄĆܱÕČŻĘ÷ĶØČėĮ½ÖÖĘųĢå·¢Éś»Æѧ·“Ó¦£¬·“Ó¦ÖŠø÷ĪļÖŹµÄĪļÖŹµÄĮæ±ä»ÆČēÓŅĶ¼ĖłŹ¾£¬¶ŌøĆ·“Ó¦µÄĶʶĻŗĻĄķµÄŹĒ
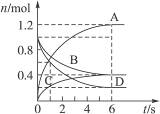
A.øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ3B+4D====6A+2C
B.·“Ó¦½ųŠŠµ½1 sŹ±£¬v(A)£½v(D)
C.·“Ó¦½ųŠŠµ½6 sŹ±£¬BµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ0.05 mol”¤(L”¤s) -1
D.·“Ó¦½ųŠŠµ½6 sŹ±£¬ø÷ĪļÖŹµÄ·“Ó¦ĖŁĀŹĻąµČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½üÄźĪŅ¹śĘū³µÓµÓŠĮæ³Ź½ĻæģŌö³¤Ē÷ŹĘ£¬Ęū³µĪ²ĘųŅŃ³ÉĪŖÖŲŅŖµÄæÕĘųĪŪČ¾Īļ”£
![]() ¢ÅĘū³µÄŚČ¼»ś¹¤×÷Ź±ŅżĘš·“Ó¦£ŗN2(g)+O2(g)
¢ÅĘū³µÄŚČ¼»ś¹¤×÷Ź±ŅżĘš·“Ó¦£ŗN2(g)+O2(g)2NO(g)£¬ŹĒµ¼ÖĀĘū³µĪ²ĘųÖŠŗ¬ÓŠNOµÄŌŅņÖ®Ņ»”£T ”ꏱ£¬ĻņČŻ»żĪŖ2 LµÄĆܱÕČŻĘ÷ÖŠ³äČė10 mol N2Óė5 mol O2£¬“ļµ½Ę½ŗāŗóNOµÄĪļÖŹµÄĮæĪŖ2 mol£¬ŌņT ”ꏱøĆ·“Ó¦µÄĘ½ŗā³£Źż
K= ”ų ”££Ø¼ĘĖć½į¹ū±£ĮōŠ”ŹżµćŗóĮ½Ī»Źż×Ö£©
¢ĘŅ»¶ØĮæµÄNO·¢Éś·Ö½āµÄ¹ż³ĢÖŠ£¬NOµÄ×Ŗ»ÆĀŹĖꏱ¼ä±ä»ÆµÄĒśĻßČēÓŅĶ¼ĖłŹ¾”£
£ØŅŃÖŖ£ŗT1<T2£©
¢Ł·“Ó¦ 2NO(g) N2(g)+O2(g)ĪŖ(Ģī”°ĪüČČ”±»ņ”°·ÅČČ”±) ”ų ·“Ó¦”£
¢ŚŅ»¶ØĪĀ¶ČĻĀ£¬Äܹ»ĖµĆ÷·“Ó¦ 2NO(g) N2(g)+O2(g) ŅŃ“ļµ½Ę½ŗāµÄŹĒ£ØĢīŠņŗÅ£©”ų”£
a£®ČŻĘ÷ÄŚµÄŃ¹Ēæ²»·¢Éś±ä»Æ b£®NO·Ö½āµÄĖŁĀŹŗĶNOÉś³ÉµÄĖŁĀŹĻąµČ
c£®NO”¢N2”¢O2µÄÅØ¶Č±£³Ö²»±ä d£®µ„Ī»Ź±¼äÄŚ·Ö½ā4 mol NO£¬Ķ¬Ź±Éś³É2 mol N2
¢Ē¢Łµ±·¢¶Æ»ś²ÉÓĆĻ”±”Č¼ÉÕŹ±£¬Ī²ĘųÖŠµÄÖ÷ŅŖĪŪČ¾ĪļĪŖNOx£¬æÉÓĆCxHy£ØĢž£©“߻ƻ¹ŌNOxĻū³żµŖŃõ»ÆĪļµÄĪŪČ¾”£
ŅŃÖŖ£ŗCH4(g)£«4NO2(g) £½ 4NO(g)£«CO2(g)£«2H2O(g) ”÷H1£½£574 kJ”¤mol-1
CH4(g)£«4NO(g) £½ 2N2(g)£«CO2(g)+2H2O(g) ”÷H2
CH4(g)£«2NO2(g) £½ N2(g)£«CO2(g)£«2H2O(g) ”÷H3£½£867 kJ”¤mol-1
”÷H2£½ ”ų ”£
¢ŚŹ¹ÓĆ“ß»Æ¼ĮæÉŅŌ½«Ęū³µĪ²ĘųµÄÖ÷ŅŖÓŠŗ¦³É·ÖŅ»Ńõ»ÆĢ¼(CO)ŗĶµŖŃõ»ÆĪļ(NOx)×Ŗ»ÆĪŖĪŽ¶¾ĘųĢ壬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”ų ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011½ģ½ĖÕŹ”Ģ©ÖŻÖŠŃ§øßČżÉĻѧʌ9ŌĀŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
½üÄźĪŅ¹śĘū³µÓµÓŠĮæ³Ź½ĻæģŌö³¤Ē÷ŹĘ£¬Ęū³µĪ²ĘųŅŃ³ÉĪŖÖŲŅŖµÄæÕĘųĪŪČ¾Īļ”£
|
 2NO(g)£¬ŹĒµ¼ÖĀĘū³µĪ²ĘųÖŠŗ¬ÓŠNOµÄŌŅņÖ®Ņ»”£T ”ꏱ£¬ĻņČŻ»żĪŖ2 LµÄĆܱÕČŻĘ÷ÖŠ³äČė10 mol N2Óė5 mol O2£¬“ļµ½Ę½ŗāŗóNOµÄĪļÖŹµÄĮæĪŖ2 mol£¬ŌņT ”ꏱøĆ·“Ó¦µÄĘ½ŗā³£ŹżK= ”ų ”££Ø¼ĘĖć½į¹ū±£ĮōŠ”ŹżµćŗóĮ½Ī»Źż×Ö£©
2NO(g)£¬ŹĒµ¼ÖĀĘū³µĪ²ĘųÖŠŗ¬ÓŠNOµÄŌŅņÖ®Ņ»”£T ”ꏱ£¬ĻņČŻ»żĪŖ2 LµÄĆܱÕČŻĘ÷ÖŠ³äČė10 mol N2Óė5 mol O2£¬“ļµ½Ę½ŗāŗóNOµÄĪļÖŹµÄĮæĪŖ2 mol£¬ŌņT ”ꏱøĆ·“Ó¦µÄĘ½ŗā³£ŹżK= ”ų ”££Ø¼ĘĖć½į¹ū±£ĮōŠ”ŹżµćŗóĮ½Ī»Źż×Ö£© 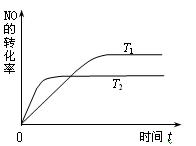
 N2(g)+O2(g)ĪŖ(Ģī”°ĪüČČ”±»ņ”°·ÅČČ”±) ”ų ·“Ó¦”£
N2(g)+O2(g)ĪŖ(Ģī”°ĪüČČ”±»ņ”°·ÅČČ”±) ”ų ·“Ó¦”£ N2(g)+O2(g) ŅŃ“ļµ½Ę½ŗāµÄŹĒ£ØĢīŠņŗÅ£©”ų ”£
N2(g)+O2(g) ŅŃ“ļµ½Ę½ŗāµÄŹĒ£ØĢīŠņŗÅ£©”ų ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ½ĖÕŹ”¶«ĢØŹŠĢĘŃó֊ѧø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
Ęū³µÄŚČ¼»ś¹¤×÷Ź±²śÉśµÄøßĪĀ»įŅżĘšN2ŗĶO2 ·¢Éś·“Ӧɜ³ÉNOĘųĢ壬Ęä·½³ĢŹ½ĪŖ£ŗ N2 (g) £«O2(g) 2NO (g)£¬øĆ·“Ó¦ŹĒµ¼ÖĀĘū³µĪ²ĘųÖŠŗ¬ÓŠNOµÄŌŅņÖ®Ņ»”£
2NO (g)£¬øĆ·“Ó¦ŹĒµ¼ÖĀĘū³µĪ²ĘųÖŠŗ¬ÓŠNOµÄŌŅņÖ®Ņ»”£
£Ø1£©ÓŅĶ¼±ķŹ¾ŌŚT1”¢T2Į½ÖÖ²»Ķ¬ĪĀ¶ČĻĀ£¬Ņ»¶ØĮæµÄNO·¢Éś·“Ó¦£ŗ2NO(g) N2(g)£«O2(g)”£·“Ó¦¹ż³ĢÖŠN2µÄĢå»ż·ÖŹżĖꏱ¼ä±ä»ÆµÄĶ¼Ļń”£ÉżøßĪĀ¶Č£¬Ōņ·“Ó¦N2 (g)£«O2(g)
N2(g)£«O2(g)”£·“Ó¦¹ż³ĢÖŠN2µÄĢå»ż·ÖŹżĖꏱ¼ä±ä»ÆµÄĶ¼Ļń”£ÉżøßĪĀ¶Č£¬Ōņ·“Ó¦N2 (g)£«O2(g) 2NO (g)µÄĘ½ŗā³£ŹżK½« £ØĢī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±£©”£
2NO (g)µÄĘ½ŗā³£ŹżK½« £ØĢī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±£©”£
£Ø2£©2000”ꏱ£¬ĻņČŻ»żĪŖ2 LµÄĆܱÕČŻĘ÷ÖŠ³äČė10 mol N2Óė5 mol O2£¬“ļµ½Ę½ŗāŗóNOµÄĪļÖŹµÄĮæĪŖ2 mol£¬Ōņ2000”ꏱ·“Ó¦N2 (g)£«O2(g) 2NO (g)µÄĘ½ŗā³£ŹżKµÄŹżÖµĪŖ ”£øĆĪĀ¶ČĻĀ£¬ČōæŖŹ¼Ź±ĻņÉĻŹöČŻĘ÷ÖŠ³äČėN2ÓėO2¾łĪŖ1 mol£¬Ōņ“ļµ½Ę½ŗāŗóN2µÄ×Ŗ»ÆĀŹĪŖ ”£
2NO (g)µÄĘ½ŗā³£ŹżKµÄŹżÖµĪŖ ”£øĆĪĀ¶ČĻĀ£¬ČōæŖŹ¼Ź±ĻņÉĻŹöČŻĘ÷ÖŠ³äČėN2ÓėO2¾łĪŖ1 mol£¬Ōņ“ļµ½Ę½ŗāŗóN2µÄ×Ŗ»ÆĀŹĪŖ ”£
£Ø3£©ŃŠ¾æ·¢ĻÖ£¬ÓĆCH4“߻ƻ¹ŌNOxæÉŅŌĻū³żµŖŃõ»ÆĪļµÄĪŪČ¾”£ĄżČē£ŗ
CH4(g)£«4NO2(g)£½4NO(g)£«CO2(g)£«2H2O(g) ”÷H1£½£574 kJ”¤mol£1
CH4(g)£«4NO(g)£½2N2(g)£«CO2(g)£«2H2O(g) ”÷H2£½ØD1160 kJ”¤mol£1
Čō1 mol CH4»¹ŌNO2ÖĮN2£¬Õūøö¹ż³ĢÖŠ·Å³öµÄČČĮæĪŖ kJ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com