
ij�����ܱ������У����淴ӦA(s)B��C(g)����H����Q kJ��mol��1(Q��0)�ﵽƽ�⡣��С������������´ﵽƽ��ʱ��C(g)��Ũ������С���ǰ��ƽ��Ũ����ȡ����·�����ȷ����(����)
A������B��״ֻ̬��Ϊ��̬��Һ̬
B��ƽ��ʱ����λʱ����n(A)��Ӧ��n(C)������1��1
C������ʼʱ�������м���1 mol B��1 mol C���ﵽƽ��ʱ�ų�����Q kJ
D������������䣬��ƽ����ϵ�м���B��ƽ��������淴Ӧ�����ƶ�
 �ο�������ϵ�д�
�ο�������ϵ�д� ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)25 ��ʱ���ô�����Һ�ζ���Ũ��NaOH��Һ��pH��7��V���VNaOH(����)
(2013·�������ۣ�2B)
(2)ϡ�����ˮϡ�ͣ�����ĵ���̶�������Һ��pH��С(����)
(2012·�������ۣ�10B)
(3)�к͵�����������ʵ���Ũ�ȵ�����ʹ��������ĵ�n(NaOH)���(����)
(2012·�������ۣ�10C)
(4)CH3COOH��Һ��ˮϡ�ͺ���Һ��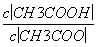 ��ֵ��С(����)
��ֵ��С(����)
(2013·���գ�11C)
(5)�����£�����0.10 mol·L��1�İ�ˮ����ˮϡ�ͺ���Һ��c(NH )·c(OH��)���(����)
)·c(OH��)���(����)
(2013·�������ۣ�8B)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼������������ѧ��ѧ��Ҫ�ķǽ���Ԫ�أ��ڹ�ũҵ�������й㷺��Ӧ�á�
 ��1�����ڷ��䡰�칬һ�š��ij������Ż����ȼ����Һ̬ƫ�����£�CH3��2N��NH2����������Һ̬�����������������ڷ�Ӧ�����зų�����������ͬʱ������������Ⱦ�����塣��֪�����£�1 gȼ����ȫȼ���ͷų�������Ϊ42.5kJ����д���÷�Ӧ���Ȼ�ѧ����ʽ________________________________________��
��1�����ڷ��䡰�칬һ�š��ij������Ż����ȼ����Һ̬ƫ�����£�CH3��2N��NH2����������Һ̬�����������������ڷ�Ӧ�����зų�����������ͬʱ������������Ⱦ�����塣��֪�����£�1 gȼ����ȫȼ���ͷų�������Ϊ42.5kJ����д���÷�Ӧ���Ȼ�ѧ����ʽ________________________________________��
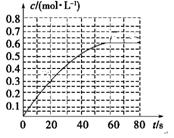
��2��298 Kʱ����2L���ܱ������У��������淴Ӧ��2NO2(g)
N2O4(g)����H����a kJ��mol��1 (a��0) ��N2O4�����ʵ���Ũ����ʱ���
����ͼ����ƽ��ʱ�� N2O4��Ũ��ΪNO2��2�����ش��������⡣
��298kʱ���÷�Ӧ��ƽ�ⳣ��Ϊ________��
�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����
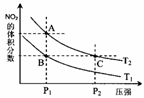 a��A��C����ķ�Ӧ���ʣ�A��C
a��A��C����ķ�Ӧ���ʣ�A��C
b��B��C����������ƽ����Է���������B��C
c��A��C�����������ɫ��A�Cdz
d����״̬B��״̬A�������ü��ȵķ���
������Ӧ��398K���У�ijʱ�̲��n��NO2��=0.6 mol n��N2O4��=1.2mol�����ʱV������ V���棩���>������<����=������
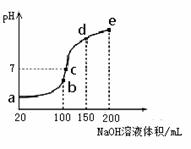 ��3��NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺������100 mL 0.1 mol��L��1NH4HSO4��Һ�еμ�0.1 mol��L��1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��
��3��NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺������100 mL 0.1 mol��L��1NH4HSO4��Һ�еμ�0.1 mol��L��1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��
�Է���ͼ��a��b��c��d��e����㣬
��ˮ�ĵ���̶�������__________��
������Һ��c(OH-)����ֵ��ӽ�NH3��H2O�ĵ��볣��K��ֵ���� ��
����c�㣬��Һ�и�����Ũ���ɴ�С������˳����_______ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����(�� ��)
A.ԭ�Ӿ����еĸ�����ԭ�Ӷ��Թ��ۼ�����
B.���������ԭ��������糡�����½����������ɵ���,���Ӷ����˶�
C.���Ӿ�����۷е�ܵ�,�����¶���Һ̬����̬
D.���Ӿ�����ÿ��������Χ��������6�����෴��ɵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
C60�����ʯ��ʯī��������̼���Ȼ�蘆Ľṹģ����ͼ��ʾ(ʯī����ʾ�����е�һ��ṹ):
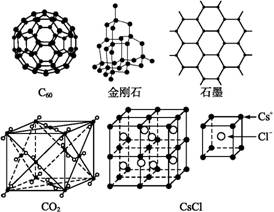
(1)C60�����ʯ��ʯī���ߵĹ�ϵ�ǻ�Ϊ����������
A.ͬ���칹�� B.ͬ�������� C.ͬϵ�� D.ͬλ��
(2)��̬ʱ��C60���ڡ������� (�ԭ�ӡ����ӡ�)���壬C60�����к���˫������Ŀ�ǡ���������
(3)�����Ľṹ�����ʯ����,1 mol������к��й衪�赥������Ŀ�ǡ�������NA��
(4)ʯī��״�ṹ��,ƽ��ÿ����������ռ�е�̼ԭ�����ǡ����� ����
(5)�۲�CO2���Ӿ���ṹ��һ����,��˵��ÿ��CO2������Χ�С����� ������֮�����ҵȾ��CO2
���ӣ��ýṹ��Ԫƽ��ռ�С���������CO2���ӡ�
(6)�۲�ͼ���Ʋ�,CsCl�����������������Cs+�����Ϊa����ÿ��Cs+��Χ�������Ϊa��Cs+��ĿΪ����������ÿ��Cs+��Χ��������Ҵν���Cs+��ĿΪ��������������Ϊ���� ����ÿ��Cs+��Χ��������ҵ�������Cs+��ĿΪ��������������Ϊ������ ����ÿ��Cs+��Χ�����ҵȾ��Cl-��ĿΪ������ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�������£���E(g)��F(g)������������2 L�ܱ������У�����������Ӧ������5 minĩ�ﵽƽ�⣺2E(g)��F(g)2G(g)���й��������£�
| E(g) | F(g) | G(g) | |
| ��ʼŨ��/mol��L��1 | 2.0 | 1.0 | 0 |
| ƽ��Ũ��/mol��L��1 | c1 | c2 | 0.4 |
�����ж���ȷ����(����)
A����Ӧ��ǰ5 min�ڣ�v(E)��0.04 mol��L��1��min��1
B�������������䣬������E��Ũ�ȣ����ƽ��ʱE��ת���ʻ�����
C�������������䣬�����¶ȣ�ƽ��ʱn(E)��3.0 mol����Ӧ�Ħ�H��0
D��ƽ�������2.0 mol E��1.0 mol F������ͬ�������ٴ�ƽ��ʱ��c(G)��0.2 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ����д�缫��Ӧʽ���ܷ���ʽ
(1)�ö��Ե缫���AgNO3��Һ��
������Ӧʽ_______________________________________________________��
������Ӧʽ________________________________________________________��
�ܷ�Ӧ���ӷ���ʽ__________________________________________________________��
(2)�ö��Ե缫���MgCl2��Һ
������Ӧʽ________________________________________________________________��
������Ӧʽ_________________________________________________________________��
�ܷ�Ӧ���ӷ���ʽ___________________________________________________________��
(3)�������缫���NaCl��Һ
������Ӧʽ__________________________________________________________________��
������Ӧʽ_________________________________________________________________��
�ܷ�Ӧ��ѧ����ʽ______________________________________________________________��
(4)�������缫���NaOH��Һ
������Ӧʽ_________________________________________________________________��
������Ӧʽ________________________________________________________________��
�ܷ�Ӧ���ӷ���ʽ______________________________________________________________��
(5)��ͭ���缫���������Һ
������Ӧʽ_________________________________________________________________��
������Ӧʽ_________________________________________________________________��
�ܷ�Ӧ���ӷ���ʽ______________________________________________________________��
(6)��Al���缫���NaOH��Һ
������Ӧʽ_________________________________________________________________��
������Ӧʽ_________________________________________________________________��
�ܷ�Ӧ���ӷ���ʽ______________________________________________________________��
(7)������Ϊ���������H2SO4��Һ�����ı����γ�����Ĥ
������Ӧʽ_______________________________________________________________��
������Ӧʽ_______________________________________________________________��
�ܷ�Ӧ���ӷ���ʽ______________________________________________________________��
(8)��Al������������ʯī�����������NaHCO3��Һ
������Ӧʽ_______________________________________________________________��
������Ӧʽ_______________________________________________________________��
(9)�ö��Ե缫�������MgCl2
������Ӧʽ__________________________________________________________________��
������Ӧʽ_________________________________________________________________��
�ܷ�Ӧ���ӷ���ʽ_______________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com