
������[KAl(SO4)2��12H2O]�Ʊ�Al��K2SO4��H2SO4���������£�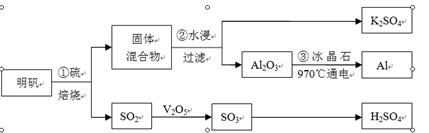
�������յĻ�ѧ����ʽΪ��4KAl(SO4)2��12H2O+3S��2K2SO4 +2Al2O3+9SO2��+48H2O
��ش��������⣺
��1���ڱ��������ķ�Ӧ�У��������� ��
��2��������У�Ϊ��߽����ʣ��ɲ�ȡ�Ĵ�ʩ�� ��
| A������������� | B�������¶� | C�����Ͻ��� | D�����̽���ʱ�� |
��1��KAl(SO4)2��12H2O��2�֣�
��2�� AC ��2�֣���ѡ����ѡ0�֣�
��3�������ᾧ��2�֣�������Ũ������ȴ�ᾧ����1�֣�
��4��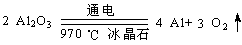 ��2�֣�
��2�֣�
������̼��������CO2��CO�� ��2�֣��û�ѧ����ʽ��ʾҲ�ɣ��磺
C��O2 CO2��2C��O2
CO2��2C��O2 2CO �������ü��Ȼ�970��Ҳ�ɣ�
2CO �������ü��Ȼ�970��Ҳ�ɣ�
��5��NiO(OH)+H2O��e����Ni(OH)2��OH����2�֣�http://w ww.xkb1��com
��6�� ��
��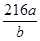 ��3�֣�������λ���۷֣�����Ҳ���ԣ�
��3�֣�������λ���۷֣�����Ҳ���ԣ�
���������������1�������ı����Ѿ������˷���ʽ����˱�����Ҫ�ǿ���������ԭ��Ӧ���������÷���ʽ�з������ϼ۱仯��ֻ��SԪ�أ������е�SԪ��һ���ַ������ϼ۽��ͣ���S���ʵĻ��ϼ����ߣ���˷�Ӧ�е���������������KAl(SO4)2��12H2O������ԭ���ǵ���S��
��2���������ˮ���������պ�Ĺ���������Ҫ��߽����ʣ�������Ҫ����Ӵ����������ܽ⣻��˷������������Ͻ��趼�ǿ��еġ�
��3�����պ�Ĺ���������K2SO4��Al2O3��ˮ�������Һ����������ˮ��K2SO4��Һ������Ҫ�ӵ�һ��ֵ���Һ�еõ������ʣ�����ֱ�������ᾧ��
��4������������Ʊ������ʣ��÷�Ӧ����ʽΪ ���ڵ�ⷴӦ��������ԭ�õ��������������õ�O2�����������缫������C���ʣ������ڸ��»����£�̼�ز��ϻᱻ��������ΪCO��CO2���Ӷ���������̼�ص缫����Ҫ���ڸ�����
���ڵ�ⷴӦ��������ԭ�õ��������������õ�O2�����������缫������C���ʣ������ڸ��»����£�̼�ز��ϻᱻ��������ΪCO��CO2���Ӷ���������̼�ص缫����Ҫ���ڸ�����
��5����Al��NiO(OH)Ϊ�缫���ɵļ��Ե�أ������жϳ�����Al��������NiO(OH)Ϊ��������˷ŵ�ʱ�����õ����ӱ���ԭ����Ni�Ļ��ϼ۱仯���Եó���ʧ������Ŀ�����Գ�����õ���غ㡢ԭ���غ㣬�ٽ�Ϸ�Ӧ�������Եĸ������ķ�ӦʽΪNiO(OH)+H2O��e����Ni(OH)2��OH����
��6������˼·�������������ʵ��������ݷ���ʽ����SO2�������ʵ���������Sԭ���غ�H2SO4���ʵ����ʵ�������96%��SO2��������H2SO4�����ʵ���ת��Ϊ��������������H2SO4���������������������H2SO4��Һ����������������ʽΪ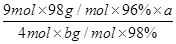 ��
��
���㣺���⿼����DZȽϼĻ�ѧ���������⡣


 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������Դ�������ȷ������Ź㷺����;��ʪ�����ɷ��Ʊ��������ε�ԭ�����±���ʾ��
��1����ҵ����ʪ���Ʊ�������أ�K2FeO4����������ͼ��ʾ��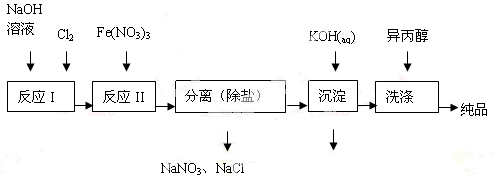
��ϴ�Ӵ�Ʒʱѡ�������������ˮ�������� ��
�ڷ�ӦII�����ӷ���ʽΪ ��
�۸��������ˮ�м�������ɱ�������ܾ�ˮ����һ�������ˮ����������������ɱ������Ϊ ���ܾ�ˮ��ԭ���� ��
����֪25��ʱFe(OH)3��Ksp = 4.0��10-38����ӦII�����Һc(Fe3+)=4.0��10-5mol/L,����Ҫ������ ʱ����ʼ����Fe(OH)3����������Һ����ı仯����
��2��������ͼ�ɼ���ʪ���Ʊ��������ʱ�������Ƶø������ƣ�Ȼ��������������м��뱥��KOH��Һ����������������ء�
�ټ��뱥��KOH��Һ��Ŀ���� ��
����������Ϣ��֪��������ص��ܽ�ȱȸ������� �����С������
| ʪ�� | ǿ���Խ����У�Fe(NO3)3��NaClO��Ӧ�����Ϻ�ɫ����������Һ |
| �ɷ� | Fe2O3��KNO3��KOH��ϼ��ȹ��������Ϻ�ɫ�������κ�KNO2�Ȳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��֪A��B���������ֶ�����Ԫ����ɵĻ����A��ijԪ�ص���������Ϊ25%��B����ɫ��Ӧ�ʻ�ɫ��C��J��X��ͬ���ڵ�Ԫ�صļ��⻯�XΪ��ɫҺ�壬C��JΪ���壬D��һ�ֲ�����ˮ�İ�ɫ���塣��Ӧ���ɵ�ˮ������ȥ������������ͼ��ʾ�Ĺ�ϵ��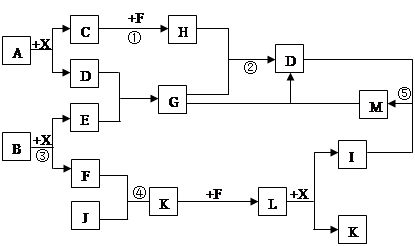
��1��д����ѧʽ��A ,E ,L ��
��2���ڷ�Ӧ�٢ڢۢܢ�������������ԭ��Ӧ���� ��
��3����Ӧ�ۻ�ѧ����ʽΪ�� ��
��4��д���������ӷ���ʽ����Ӧ�� ��
G��Һ��M��Һ�ķ�Ӧ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��A��B���ֳ�����������ɵĻ�������ɫ��Ӧ��Ϊ��ɫ�����ת����ϵ��ͼ(��
�����ʾ���ȥ)��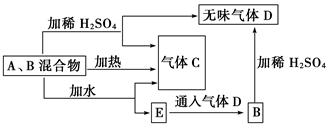
�������Ϲ�ϵ���ش��������⣺
(1)д��A��B��C��D�Ļ�ѧʽ��A________��B________��C________��D________��
(2)д��������м�ˮ��Ӧ�Ļ�ѧ����ʽ��_____________________________________
(3)���Ⱥ���ֻ�õ�һ�ֹ��廯�����A��B�����ʵ���֮�ȵ����ֵΪ________(������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��FeCl3��Һʴ��ͭ�������·��Ĺ����У���Һ��������Դ���յĹ��̼������£�
��.���Һ��Ͷ�������м����ַ�Ӧ�������������Һ��
��.����Һ�м���һ����ʯ��ˮ��������ҺpH��ͬʱ���������Ŀ�����
��֪��Ksp[Fe(OH)3]��4.0��10��38
�ش��������⣺
(1)FeCl3ʴ��ͭ����Ӧ�����ӷ���ʽΪ______________________________��
(2)���̢������м����Ҫ������________������õ��������Ҫ�ɷ���______________���ӹ����з����ͭ����õķ�����________________��
(3)���̢��з�����Ӧ�Ļ�ѧ����ʽΪ______________��
(4)���̢��е�����Һ��pHΪ5����������Ũ��Ϊ________��(��ʽ����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ��������Ĺ�������ʾ��ͼ���£�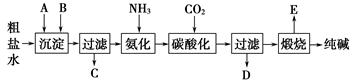
���������գ�
(1)����ˮ���������A��B������(������A��Դ��ʯ��Ҥ��)��д��A��B�Ļ�ѧʽ��
A______________��B____________��
(2)ʵ�����ᴿ���ε�ʵ���������Ϊ��
ȡ����__________��������__________��__________����ȴ�ᾧ��__________����ɡ�
(3)��ҵ��������������У�̼�ữʱ������������__________________��
̼�ữʱû������̼���ƾ��壬��ԭ����______________________��
(4)̼�ữ����ˣ���ҺD����Ҫ�ijɷ���______________(��д��ѧʽ)��������һ�ɷֵ������ӵľ��巽���ǣ�______________________��
(5)��������а���ѭ��ʹ�õģ�Ϊ�ˣ���ҺD����ʯ��ˮ����������ʯ��ˮ���������ķ�Ӧ�����ӷ���ʽΪ��____________________________��
(6)��Ʒ�����к���̼�����ơ�����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ����������ɱ�ʾΪ��__________(ע����ı���ʽ�����õ��йط��ŵĺ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���ô�����ͭ(������FeO)Ϊԭ����ȡ�Ȼ�ͭ����(CuCl2��2H2O)�������������£�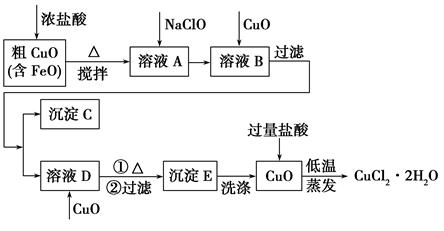
| ���� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| ��ȫ����ʱ��pH | ��9.6 | ��6.4 | 3��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͭ����Ҫ�Ľ������ϡ�
(1)��ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ���÷�Ӧ��������Ϊ________������ͭ��ȡ��ͭ�����ʱ������������________�����Һ�б��뺬�е���������________��
(2)��100 mL 18 mol��L��1Ũ�����м��������ͭƬ������ʹ֮��ַ�Ӧ����Ӧ�б���ԭ��H2SO4Ϊ________mol��
(3)���ӹ�ҵ������������Ϊ30%��FeCl3��Һ��ʴ����ͭ���ľ�Ե����ӡˢ��·�壬Ϊ�˴�ʹ�ù��ķϸ�ʴҺ�л���ͭ�������µõ�FeCl3��Һ���������ʵ�����̡�
���������У������Լ��Ļ�ѧʽΪ��X________��Y________��Z________���ڢ���Ӧ�����ӷ���ʽΪ
___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������������������������Ҫ��Ӧ�ã�������ʹ�ø���IJ��Ǵ����������ǺϽ�
��1�������ӷ�Ӧ�ѵ��Ƚ������õ����ƼغϽ��䳣������ ̬��������Һ���̣������Ƶ����ʵ�������Ϊ20%��0.2mol�Ĵ˺Ͻ�ȫ�ؼ��뵽��ˮ��D2O���У���������������������������Ϊ ��
��2��þ���Ͻ��Ǿ������������ʺϽ𡣼�һ��Ͻ��ڿ�����ȼ�գ�������MgO��Al2O3�⣬���п������ɵĵ��������ʵĵ���ʽ�� ����һ��5.1g��þ���Ͻ�Ƭ����3.6 mol��L-1��200ml ��������Һ�У����������1 mol��L-1������������Һ����� mL�������������ٸı䣬��������������0.5mol �ĵ��ӷ���ת�ƣ���Ͻ���Mg�����ʵ�������Ϊ ��
��3������һ��ͭ�ĺϽ�ͭ���ɿ�����Cu��Zn�����ɷֱ������ܷ�����ܷ�����������ֽ��������аѸúϽ�Ͷ�뵽ϡ�����У����ֲ������ݵ��ٶȱ���п�����ᷴӦ���������ٶȿ죬��ԭ���� ��
��Ϊ����ijͭ�Ͻ�ijɷ֣����Ὣ����ȫ�ܽ����NaOH��Һ��pH����pH��3.4ʱ��ʼ���ֳ������ֱ���pHΪ7.0��8.0ʱ���˳����������ͼ��Ϣ�ƶϸúϽ��г�ͭ��һ������ ��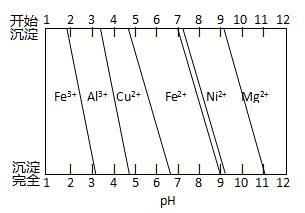
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com