


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.01mol/L�������0.01mol/L��NaOH��Һ�������� |
| B��pH=3�Ĵ����pH=11��NaOH��Һ�������� |
| C��0.01mol/L�������0.01mol/L�İ�ˮ��Һ�������� |
| D��pH=3�������pH=11�İ�ˮ��Һ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2��9 | B��1��9 | C��1��1 | D��1��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ˮ�������������Ũ�ȣ�a��b |
| B����������ʵ���Ũ��Ϊ0.0100mol?L-1?? |
| C��ָʾ����ɫʱ��˵��������NaOHǡ����ȫ��Ӧ |
| D�����μ�NaOH��Һ10.00 mL�����Է�Ӧǰ������仯�����û��Һ��pH=1+lg3 |
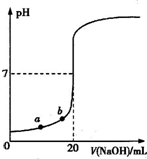
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��pH=3�Ĵ�����Һ��pH=11������������Һ�������Ϻ�pH=7 |
| B�����ʵ���Ũ����ȵ�CH3COOH��CH3COONa��Һ�������ϣ�c��CH3COO-��+c��CH3COOH��=2c��Na+�� |
| C�������£���NH4Cl��Һ�м��백ˮ����Һ��pH=7����ʱ��Һ��c��NH4+����c��Cl-�� |
| D��0.1mol?L-1 NaHCO3��Һ��c��Na+����c��HCO3-����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��32g | B��48g | C��56g | D��64g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ���� | Cl2 | Br2 | I2 | HCl | HBr | HI | H2 |
| ������kJ�� | 243 | 193 | 151 | 432 | 366 | 298 | 436 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��v��NH3��=0.02mol?L-1?s-1 |
| B��v��O2��=0.01mol?L-1?s-1 |
| C��v��N2��=0.02mol?L-1?s-1 |
| D��v��H2O��=0.02mol?L-1?s-1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com