
(1)д��˫�����Fe3����ϵ����ӷ���ʽ��________________________����ȡ������Ҫ�������˵���ȡ������Һ��pH����������________________________��
��ͼ����˫����(H2Dz)��CCl4�����ȡijЩ�������ӵ�������ߡ�����ӳ����ȡijЩ��������ʱ���˵�pH��Χ��E%��ʾij�ֽ����������������ʽ����ȡ����İٷ��ʡ�

ij��ҵ��ˮ�к���Hg2����Bi3+��Zn2+����˫����(H2Dz)��CCl4�����ȡ��������ˮ��
�������ͼ�ش����⣺
(2)����ȫ����ˮ�е�Hg2������������������ҺpH=____________��
(3)������pH=2ʱ����(Bi)�Ĵ�����ʽ��____________�������ʵ���֮��Ϊ__________��
(4)��ȡ��CCl4�е�Zn(HDz)2��Һ����������NaOH��Һ�������п��ת��ˮ��Һ�С�д����Ӧ�����ӷ���ʽ��________________________��
(1)Fe3++3H2Dz![]() Fe(HDz)3+3H+ Fe3+���γ�Fe(OH)3����
Fe(HDz)3+3H+ Fe3+���γ�Fe(OH)3����
(2)1
(3)Bi3+��Bi(HDz)3 3��2
(4)Zn(HDz)2+6OH-![]()
![]() +2Dz2-+2H2O
+2Dz2-+2H2O
������(1)��˫����ѽ���������ϳɵ����Ե�����֪��Fe3++3H2Dz![]() Fe(HDz)3+3H+���������pH�����Fe3+��ת��ΪFe(OH)3������
Fe(HDz)3+3H+���������pH�����Fe3+��ת��ΪFe(OH)3������
(2)��ͼ�ɿ���pH=1ʱHg2+ȫ�����������ʽ����ȡ���������
(3)��ͼ֪pH=2ʱ��Bi3+���������ʽBi(HZ2)3������ȡ�İٷ�����40%������n(Bi3+)��n��Bi(HZ2)3��=60%��40%=3��2��
(4)Zn(HDz)2+6OH-![]()
![]() +2Dz2-+2H2O
+2Dz2-+2H2O


 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| g | 2+ 2 |
| g | 2+ 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ̩���и��������ָ�ϰ����������ۻ�ѧ�Ծ��������棩 ���ͣ�������
��ˮ��������ʱ������˫����(H2Dz����Ԫ����)�ѽ���������ϳɵ����Ե����ʣ�����CCl4��ȡ�����Ӷ��ѽ������Ӵ�ˮ��Һ����ȫ�������������˫����(H2Dz)��CCl4������ˮ�е�Cu2+ʱ���ȷ�����Ϸ�Ӧ��Cu2++2H2DZ Cu (HDZ)2+2H+���ټ���CCl4
��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С�
Cu (HDZ)2+2H+���ټ���CCl4
��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С�
��1��д��˫�����Fe3+��ϵ����ӷ���ʽ��_____________________����ȡFe3+�Ĺ�����Ҫ�������˵���ȣ������Һ��pH����������___________________________��
��2����ͼ����˫����(HzDz)��CCl4�����ȡijЩ�������ӵ�������ߣ�����ӳ����ȡijЩ��������ʱ���˵�pH��Χ��E����ʾij�ֽ����������������ʽ��ȡ����İٷ��ʡ�
ij��ҵ��ˮ�к���Hg2+��Bi3+��Zn2+����˫���꣨H2Dz��~ CCl4�����ȡ��������ˮ��
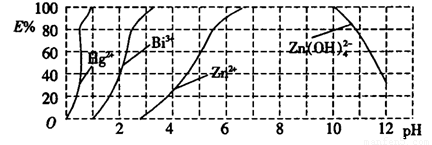
������ȫ����ˮ�е�Hg2+����������������Һ��pH=________��
�ڵ�����pH=2ʱ����(Bi)�Ĵ�����ʽ��_________________��
��3����ˮ�е��ǹ�����(Hg2+ 2)����ת���ɹ�����(Hg2+)������˫������ϡ�ij������ˮ�к��н϶���Ȼ��ǹ�(Hg2Cl2)������������(K2S2O8)������(Hg2+ 2)��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����12��������ѧ�Ծ� ���ͣ������
��12�֣���ˮ��������ʱ������˫���꣨H2Dz����Ԫ���ᣩ�ѽ���������ϳɵ����Ե����ʣ�����CCl4��ȡ�����Ӷ��ѽ������Ӵ�ˮ��Һ����ȫ�������������˫���꣨H2Dz����CCl4������ˮ�е�Cu2��ʱ���ȷ�����Ϸ�Ӧ��
Cu2��+2 H2Dz Cu(HDz)2+2H����
Cu(HDz)2+2H����
�ټ���CCl4��Cu(HDz)2�ͺ����ױ���ȡ��CCl4�С�
��1��д��˫�����Fe3����ϵ����ӷ���ʽ�� ����ȡ������Ҫ�������˵���ȡ������Һ��pH���������� ��
��ͼ����˫���꣨H2Dz����CCl4�����ȡijЩ�������ӵ�������ߡ�����ӳ����ȡijЩ��������ʱ���˵�pH��Χ��E%��ʾij�ֽ����������������ʽ����ȡ����İٷ��ʡ�

ij��ҵ��ˮ�к���Hg2����Bi3+��Zn2+����˫���꣨H2Dz����CCl4�����ȡ��������ˮ��
�������ͼ�ش����⣺
��2������ȫ����ˮ�е�Hg2������������������ҺpH=
��3��������pH=2ʱ���飨Bi���Ĵ�����ʽ�У� �������ʵ���֮��Ϊ
��4����ȡ��CCl4�е�Zn(HDz)2��Һ����������NaOH��Һ�������п��ת��ˮ��Һ�С�д����Ӧ�����ӷ���ʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com