
ijŠĖȤŠ”×éµÄĶ¬Ń§ĆĒ¹²Ķ¬Éč¼ĘĮĖĻĀĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ£¬“Ė×°ÖĆ¼ČæÉÓĆÓŚÖĘČ”ĘųĢ壬ÓÖæÉÓĆÓŚŃéÖ¤ĪļÖŹµÄijŠ©ŠŌÖŹ£®
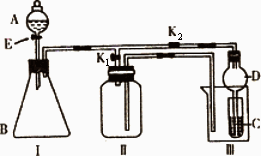
(1)ČōĄūÓĆøĆ×°ÖĆÖĘČ”²¢ŹÕ¼ÆH2»ņNH3£¬ŌŚIÖŠ¼ÓČėŅ©Ę·ŗóÓ¦øĆ½ųŠŠµÄ²Ł×÷ŹĒ________£»ČōŅŖÖĘČ”²¢ŹÕ¼ÆO2»ņNO£¬øü»»¢ńÖŠŅ©Ę·ŗ󣬲»øü»»ŅĒĘ÷£¬Ö»ŠčŅŖ×÷¼ņµ„øĽų£¬¼“æÉĶź³ÉŹµŃ飬ĘäøĽųµÄ·½·ØŹĒ________£®
(2)“ņæŖK2£¬¹Ų±ÕK1£®
¢ŁĄūÓĆøĆ×°ÖĆ½ųŠŠŹµŃ飬æÉŅŌÖ¤Ć÷ŅŌĻĀĪļÖŹµÄĖįŠŌĒæČõĖ³ŠņĪŖ£ŗHCl£¾H2CO3£¾H2SiO3£®
ÓŠĶ¬Ń§ČĻĪŖ£ŗŌŚAÖŠ¼Ó________£¬BÖŠ¼ÓCaCO3£¬CÖŠ¼Ó________(¾łĢīŠ“ĪļÖŹµÄ»ÆѧŹ½)£®¹Ū²ģµ½________µÄĻÖĻ󣬼“æÉÖ¤Ć÷£®µ«ÓŠµÄĶ¬Ń§ČĻĪŖ“ĖŹµŃéÖ¤Ć÷ĖįŠŌH2CO3£¾H2SiO3Ź±ÓŠČ±ĻŻ£¬ÄćČĻĪŖȱĻŻŹĒ________£®
¢ŚĄūÓĆøĆ×°ÖĆæÉŅŌÖĘČ”Cl2£¬²¢ŌŚ70”ꏱÓėNaOHČÜŅŗ·“Ӧɜ³ÉNaClOŗĶNaClO3£®
ŹµŃé·½°øŹĒ£ŗŌŚAÖŠ¼ÓÅØŃĪĖį£¬BÖŠ¼ÓøßĆĢĖį¼Ų£¬CÖŠ¼ÓĒāŃõ»ÆÄĘČÜŅŗ£¬ÉÕ±ÖŠ¼Ó________£®
“żCÖŠČÜŅŗĒ”ŗĆ·“Ó¦ŗó£¬ĻņĘäÖŠ¼ÓČė¹żĮæKIČÜŅŗ£¬¼ÓČė“×Ėįµ÷½ŚČÜŅŗµÄĖįŠŌ£¬“ĖŹ±Ö»ÓŠNaClO±»»¹Ō£¬Č»ŗóÓĆŅ»¶ØÅØ¶ČµÄNa2S2O3ČÜŅŗµĪ¶Ø£»¼ĢŠųĻņĘäÖŠ¼ÓČėŃĪĖį£¬µ÷½ŚČÜŅŗµÄĖįŠŌ£¬“ĖŹ±NaClO3±»»¹Ō£¬ŌŁÓĆĶ¬ÅØ¶ČµÄNa2S2O3ČÜŅŗµĪ¶Ø£®(![]()
![]() )ŹµŃé½į¹ū¼ĒĀ¼ČēĻĀ£ŗ
)ŹµŃé½į¹ū¼ĒĀ¼ČēĻĀ£ŗ

ŹŌĶعż±ķÖŠŹż¾Ż¼ĘĖćCČÜŅŗÖŠÉś³ÉµÄClO£ŗĶ![]() µÄĪļÖŹµÄĮæÖ®±Č________£®
µÄĪļÖŹµÄĮæÖ®±Č________£®

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| Cu2+/molL-1 | 0.1 | 0.01 | 0.001 | 0.0001 | 0.00001 |
| ČÜŅŗpH | 4.7 | 5.2 | 5.7 | 6.2 | 6.7 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŠĖȤŠ”×éµÄĶ¬Ń§ĆĒ¹²Ķ¬Éč¼ĘĮĖĻĀĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ£¬“Ė×°ÖĆ¼“æÉÓĆÓŚÖĘČ”ĘųĢ壬ÓÖæÉÓĆÓŚŃéÖ¤ĪļÖŹµÄijŠ©ŠŌÖŹ”£
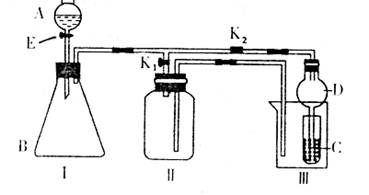
£Ø1£©ČōĄūÓĆøĆ×°ÖĆÖĘČ”²¢ŹÕ¼ÆH2»ņNH3£¬ŌŚIÖŠ¼ÓČėŅ©Ę·ŗóÓ¦øĆ½ųŠŠµÄ²Ł×÷ŹĒ
£»ČōŅŖÖĘČ”²¢ŹÕ¼ÆO2»ņNO£¬øü»»IÖŠŅ©Ę·ŗ󣬲»øü»»ŅĒĘ÷£¬Ö»ŠčŅŖ×÷¼ņµ„øĽų£¬¼“æÉĶź³ÉŹµŃ飬ĘäøĽųµÄ·½·ØŹĒ
ӣ
£Ø2£©“ņæŖK2£¬¹Ų±ÕK1”£
¢ŁĄūÓĆøĆ×°ÖĆ½ųŠŠŹµŃ飬æÉŅŌÖ¤Ć÷ŅŌĻĀĪļÖŹµÄĖįŠŌĒæČõĖ³ŠņĪŖ£ŗHC1>H2CO3>H2SiO3”£ÓŠĶ¬Ń§ČĻĪŖ£ŗŌŚAÖŠ¼Ó £¬BÖŠ¼ÓCaCO3£¬CÖŠ¼Ó £Ø¾łĢīŠ“ĪļÖŹµÄ»ÆѧŹ½£©”£¹Ū²ģµ½ µÄĻÖĻ󣬼“æÉÖ¤Ć÷”£µ«ÓŠµÄĶ¬Ń§ČĻĪŖ“ĖŹµŃéŌŚÖ¤Ć÷ĖįŠŌH2CO3>H2SiO3Ź±ÓŠČ±ĻŻ£¬ÄćČĻĪŖȱĻŻŹĒ ”£
¢ŚĄūÓĆøĆ×°ÖĆæÉŅŌÖĘČ”C12£¬²¢ŌŚ70”ꏱÓėNaOHČÜŅŗ·“Ӧɜ³ÉNaC1OŗĶNaC1O3”£ŹµŃé·½°øŹĒ£ŗŌŚAÖŠ¼ÓÅØŃĪĖį£¬BÖŠ¼ÓøßĆĢĖį¼Ų£¬CÖŠ¼ÓĒāŃõ»ÆÄĘČÜŅŗ£¬ÉÕ±ÖŠ¼Ó ”£“żCÖŠČÜŅŗĒ”ŗĆ·“Ó¦ŗó£¬ĻņĘäÖŠ¼ÓČė¹żĮæKIČÜŅŗ£¬¼ÓČė“×Ėįµ÷½ŚČÜŅŗµÄĖįŠŌ£¬“ĖŹ±Ö»ÓŠNaC1O±»»¹Ō£¬Č»ŗóÓĆŅ»¶ØÅØ¶ČµÄNa2S2O3ČÜŅŗµĪ¶Ø£»¼ĢŠųĻņĘäÖŠ¼ÓČėŃĪĖį£¬µ÷½ŚČÜŅŗµÄĖįŠŌ£¬“ĖŹ±NaC1O3±»»¹Ō£¬ŌŁÓĆĶ¬ÅØ¶ČµÄNa2S2O3ČÜŅŗµĪ¶Ø”££ØI2+2S2O32ØD=S4O62ØD+2IØD£©ŹµŃé½į¹ū¼ĒĀ¼ČēĻĀ£ŗ
| ½«KI×Ŗ»ÆĪŖI2 | µĪ¶ØI2£¬ĻūŗÄNa2S2O3ČÜŅŗµÄĢå»ż |
| KI | 5.00mL |
| KI | 30.00mL |
Ķعż±ķÖŠŹż¾Ż¼ĘĖćCČÜŅŗÖŠÉś³ÉC1OØDŗĶC1O3ØDµÄĪļÖŹµÄĮæÖ®±Č ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com