
 ���ʽṹ�����������ʣ��ش��������⣺
���ʽṹ�����������ʣ��ش��������⣺ ��������ԭ��N���ӻ���ʽ��sp3��
��������ԭ��N���ӻ���ʽ��sp3������ ��1���ٸ����������������ư�Ԫ�����ڱ����Ԫ�طֳ�5������s����p����d����f����ds�����ǽ���Ԫ�أ�����ϡ�����壩λ��p����s��������Ԫ��λ��d����ds��f����
���ӻ����ֻ�����γɦҼ�����������δ����ɼ��ŵ��ӣ�
�۷��Ӿ���Ĺ�����Ϊ���ӣ����Ӿ��������ڷ��Ӽ俿���»�������ã����γ�����������з��Ӽ仹���������
�ܾ�����Ǿ���ı��������ǣ����ڲ������ڿռ����Ƿ�һ���������������ظ����У�
������ļ�����ָX-H��Y�ij��ȣ�
��2���پ��������ֻ���Ԫ����C��N��O��HԪ�أ�Ԫ�صõ�������Խǿ����縺��Խ��
�ڶ���������ˮ����ʧȥ�ᾧˮ�Ĺ����У��ƻ��������������������
��3����ѧ�Ұ�NaNO3��Na2O��һ�������·�Ӧ�õ�һ�ְ�ɫ���壬��֪������������SO42-��Ϊ�ȵ����壬�Ҹ��������еĸ�ԭ�ӵ��������Ӷ�����8�����ȶ��ṹ������������NO43-�����ݼ۲���ӶԻ��������ж�������ԭ��N���ӻ���ʽ��
��4���ڽ��ʯ�����һ�������У�����̼ԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$+4=8��
�ڶ������辧���һ�������У�һ��Siԭ�Ӻ���4��Si-O�����þ�����Siԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$+4=8��
ԭ�Ӿ��岻���γ����ܶѻ��ṹ��ԭ����ԭ�Ӿ�����ԭ��֮�����Թ��ۼ���ϵģ����ۼ��б����Ժͷ����ԣ�һ��ԭ�Ӳ����γ�12�����ۼ���
��5��һ��Bԭ�Ӻ��ĸ�Nԭ���γ���������ṹ������Ϊ109��28�䣻�þ�����B��Nԭ�Ӹ�������4��
������������ܶ�Ϊ3.52g•cm-3���������=$\frac{\frac{25}{{N}_{A}}��4}{��}$��B-N���ļ����Ǿ����峤��$\frac{1}{4}$��
��� �⣺��1���ٸ����������������ư�Ԫ�����ڱ����Ԫ�طֳ�5������s����p����d����f����ds�����ǽ���Ԫ�أ�����ϡ�����壩λ��p����s������HԪ��λ��s��������Ԫ��λ��d����ds��f�����ʴ���
���ӻ����ֻ�����γɦҼ�����������δ����ɼ��ŵ��ӣ��ʴ���
�۷��Ӿ���Ĺ�����Ϊ���ӣ����Ӿ��������ڷ��Ӽ俿���»�������ã�����ð������������ų��������γ�����������з��Ӽ仹����������ʴ���
�ܾ�����Ǿ���ı��������ǣ����ڲ������ڿռ����Ƿ�һ���������������ظ����У��ʴ���
������ļ�����ָX-H��Y�ij��ȣ�����ȷ��
��ѡ�ݣ�
��2���پ��������ֻ���Ԫ����C��N��O��HԪ�أ�Ԫ�صõ�������Խǿ����縺��Խ�õ�������ǿ��˳����O��N��C��H�����Ե縺�Դ�С˳����O��N��C��H���ʴ�Ϊ��O��N��C��H��
�ڶ���������ˮ����ʧȥ�ᾧˮ�Ĺ����У��ƻ���������������������ʴ�Ϊ�������
��3����ѧ�Ұ�NaNO3��Na2O��һ�������·�Ӧ�õ�һ�ְ�ɫ���壬��֪������������SO42-��Ϊ�ȵ����壬�Ҹ��������еĸ�ԭ�ӵ��������Ӷ�����8�����ȶ��ṹ������������NO43-�������ʽΪ ��
��
������Nԭ�Ӽ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ�����������ԭ��N���ӻ���ʽΪ sp3��
�ʴ�Ϊ�� �� sp3��
�� sp3��
��4���ڽ��ʯ�����һ�������У�����̼ԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$+4=8��
�ڶ������辧���һ�������У�һ��Siԭ�Ӻ���4��Si-O�����þ�����Siԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$+4=8������ÿ�������й��ۼ���Ŀ��32��
ԭ�Ӿ��岻���γ����ܶѻ��ṹ��ԭ����ԭ�Ӿ�����ԭ��֮�����Թ��ۼ���ϵģ����ۼ��б����Ժͷ����ԣ�һ��ԭ�Ӳ����γ�12�����ۼ�������ԭ�Ӿ��岻���γ����ܶѻ��ṹ��
�ʴ�Ϊ��8��32��ԭ�Ӿ�����ԭ��֮�����Թ��ۼ���ϵģ����ۼ��б����Ժͷ����ԣ�һ��ԭ�Ӳ����γ�12�����ۼ�������ԭ�Ӿ���Ͳ����γ���λ����12�����ܶѻ��ṹ��
��5��һ��Bԭ�Ӻ��ĸ�Nԭ���γ���������ṹ������Ϊ109��28�䣻�þ�����B��Nԭ�Ӹ�������4��
������������ܶ�Ϊ3.52g•cm-3���������=$\frac{\frac{25}{{N}_{A}}��4}{��}$��B-N���ļ����Ǿ����峤��$\frac{1}{4}$������B
-N���ļ���=$\sqrt{3}��$$\root{3}{\frac{\frac{25��4}{{N}_{A}}}{3.52}}��\frac{1}{4}$��1010pm=$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{4��25}{3.52{N}_{A}}}$��1010pm��
�ʴ�Ϊ��109��28�䣻$\frac{\sqrt{3}}{4}$��$\root{3}{\frac{4��25}{3.52{N}_{A}}}$��1010��
���� ���⿼�����ʽṹ�����ʣ��漰�������㡢ԭ���ӻ���ʽ�жϡ��縺�ԡ��ȵ������֪ʶ�㣬���ؿ���������㼰�ռ������������������վ������㷽�����۲���ӶԻ������ۣ��ѵ��Ǿ������㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �����ʵ�����ˮ����ˮ���е������� | |
| B�� | ����������ϩ�ͱ�ϩ�к��еĹ��е��Ӷ��� | |
| C�� | ͬ�¡�ͬѹ��ͬ�����CO��NO���е������� | |
| D�� | �����ʵ������������ֱ�������������ȫ��Ӧʱת�Ƶĵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ���ȷ�ʽ | ����Ԫ����� | ��Ԫ�ص���������% | |
| Fe | O | ||
| �ƾ��� | Fe��O | 74.50 | 25.50 |
| �����־ƾ��� | Fe��O | 76.48 | 23.52 |
| �ƾ���� | Fe | 100.00 | 0.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʯ���ѽ���ȡ | B�� | ͨ���Ӿ۷�Ӧ���Ƶñ���Ĥ | ||
| C�� | ͨ���ӳɷ�Ӧ���Ƶ�����ϩ | D�� | ��ʹ��ˮ�����Ը��������Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
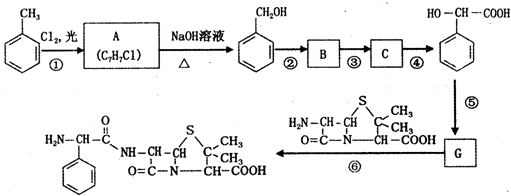

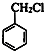 ��C
��C ��
��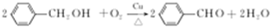 ��
��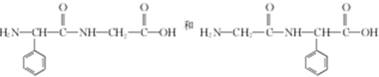 ��
�� ������������ͬ���칹��Ľṹ��ʽ��
������������ͬ���칹��Ľṹ��ʽ�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
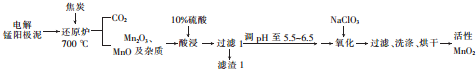
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=1����Һ�У�K+��Cr2O72-��C6H5OH��CO32- | |
| B�� | c��H+��=1��10-13mol/L����Һ�У�Cu2+��Na+��Cl-��SO42- | |
| C�� | 0.1 mol/L NH4HCO3��Һ�У�K+��Na+��Cl-��NO3- | |
| D�� | 0.1 mol/L Na2SiO3��Һ�У�K+��Cl-��NO3-��CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com