
ij����С������ȡ�������ƺ��Ȼ��ƵĻ����Һ��Ϊ��ߴ������ƺ���������ͼ��ʾװ�ã�ͼ��ƿ��ʢ����ʳ��ˮ��ƿ��ʢŨ���ᣬ��Һ©��A��ʢŨ���ᣮ(��������ʾ��Cl2��NaOH�ڲ�ͬ�¶��£����ﲻͬ���ڽϸ��¶���������NaClO3)��
�Իش�
(1)��ƿB��ʢ ���Թ�C��ʢ .
(2)��ͬѧ��Ϊ����ʡȥijЩװ�ã�����Ϊ������
�ܷ�ʡȥ��װ�ã� (��ܡ����ܡ�)��������
(3)��ͬѧ��Ϊ���������ijЩװ�ã�����Ϊ������ (���Ҫ������Ҫ��)���������Ϊ��Ҫ����ָ����װ�õ�����
(4)��װ���б�ˮ�������� ��
(1)MnO2��1�֣� NaOH��Һ ��1�֣�
(2)���� ��1�֣�HCl�������C�У�����NaOH������NaClO�ĺ�����2�֣�
(3)��Ҫ ��1�֣�Ӧ����β������װ�ã���ֹCl2��Ⱦ������2�֣�
(4)��ֹCl2��NaOH��Һ���¶Ƚϸ�ʱ������������Ӧ��2�֣�
���������������1����ȡCl2��ҪŨ������MnO2��Ӧ���Թ�C��ʢNaOH��Һ��
��2����װ���ܳ�ȥCl2��HCl�����ȥ���Ļ���HCl����C�е�NaOH��Һ������NaClO�ĺ�����
��3��Cl2�ж�������Ⱦ����������Ӧ��β������װ�ã���ֹCl2��Ⱦ������
��4�������������Ϣ����Cl2��NaOH�ڲ�ͬ�¶��£����ﲻͬ���ڽϸ��¶���������NaClO3�������Ա�ˮ�����¶ȣ���ֹ����NaClO3��
���㣺���⿼��ʵ������ͷ�����


 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ����С��ͨ��ʵ���о�NO2�����ʣ�
��֪��2NO2+2NaOH�TNaNO3+NaNO2+H2O
����ͼ1��ʾװ��̽��NO2�ܷ�NH3��ԭ��K1��K2Ϊֹˮ�У��г̶ֹ�װ����ȥ��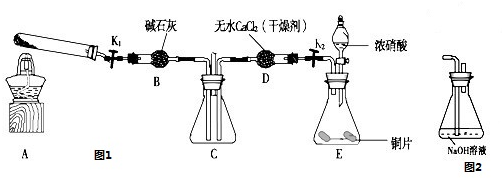
��1��Eװ������ȡNO2��Ӧ�����ӷ���ʽ��
��2����ʵ������ȡ����ʱ��ֻ��һ���Լ���������������ѡȡ �� ��
a��NH4HCO3 b��NH4Cl c��Ũ��ˮ
��3����NO2�ܹ���NH3��ԭ��Ԥ�ڹ۲쵽Cװ���е�������
��4����ʵ��װ�ô���һ�����Ե�ȱ����
��5��̽��NO2�ܷ���Na2O2����������ԭ��Ӧ��Ϊ����֤NO2�ܱ�Na2O2��������С��ͬѧѡ��B��D��Eװ�ã���B�е�ҩƷ����ΪNa2O2����ѡFװ�ã���ͼ2��ʾ����������װ������ʵ�飮װ�õĺ�������˳����
��6��ʵ������У�Bװ���е���ɫ��ĩ�� ����ɰ�ɫ�������飬�ð�ɫ����Ϊ��������������������ɣ��Ʋ�Bװ���з�Ӧ�Ļ�ѧ����ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼΪʵ������ȡ�����������Cl2�������м���Cl2����ʵ���װ�á�����Eƿ�з�
�и����ɫ������F��Ϊͭ�����Ҷ�Ϊһ������
|
|
 �Իش�
�Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧС��������װ�ó�ȡ�ռ����������������о������ʡ�������������⡣
��1��װ��A�з�����Ӧ�����ӷ���ʽΪ_______________________________��
��2��������������������ӿڵ�����˳��Ϊa��___________________��g��
��3��װ��B��Ũ�����������__________________________��װ��C���Լ������___________________________________��
��4��ijͬѧ��Ϊ��������ȱ��β������װ�ã���������ķ����л�����װ�ò�ע���Լ���
| |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ȤС����ʵ������ͭ������Ϊԭ�ϣ����ö��ַ�����ȡ����ͭ���Ʊ��������£�
����һ
�ż�ͬѧȡ6.4 gͭƬ��10 mL 18 mol��L��1Ũ���ᣬ�����Թ��й���ʱ���֣�ͭ���ȵ�Ũ���ᷴӦ��û�еõ�Ԥ�ڵ���ɫ��Һ���������Թܵײ�������ɫ��������ͬѧΪ����֤���а�ɫ��������Ҫ�ɷ֣��������ʵ�顣
ʵ�鲽�裺�㵹���ϲ�Һ��������ð�ɫ�Ĺ����м�����������ˮ���ӱ߽��衣
ʵ������ɫ�����ܽ⣬��Һ��Ϊ��ɫ��
ʵ����ۣ����ð�ɫ����Ļ�ѧʽΪ ��
��2����ͬѧ���ͬѧ����ͬ��ʵ�飬���۲쵽���ȹ����У��Թ��ڱ��ϲ�������������ɫ�������ʣ��������ȣ�����ɫ��������������������Ũ�������ʧ��ͬʱ������ʹƷ����Һ��ɫ�����壬����ɫ������ʧ��ԭ����(�û�ѧ��Ӧ����ʽ�ش�) ��ֱ�����Ӧ��ϣ������Թ��л���ͭƬʣ�ࡣ
������
��3����ͬѧ��Ϊ����Ƶ�ʵ�鷽�����ã����Լ���Ƶ�˼·�ǣ�2Cu��O2 2CuO��CuO��H2SO4��CuSO4��H2O��
2CuO��CuO��H2SO4��CuSO4��H2O��
�Աȼķ���������Ϊ��ͬѧ���ŵ��Ǣ�_________________________����_ ��
������
�ȶ�ͬѧȡһͭƬ��ϡ��������Թ��У��������е���˫��ˮ��������Һ����ɫ��д����Ӧ�Ļ�ѧ��Ӧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ̽��ij��̼�Ͻ���Ũ�����ڼ��������µķ�Ӧ�IJ��ֲ��ﲢ�ⶨ��̼�Ͻ�����Ԫ�ص�����������ij��ѧ�С���������ͼ��ʾ��ʵ��װ�ã����������ʵ��̽����
|
|

 ��1����Բ����ƿ�м���m g��̼�Ͻ𣬲��������Ũ���ᣬδ��ȼ�ƾ���ǰ��A��B��������������ԭ���ǣ��ٳ����£�Fe��Ũ�����жۻ����� ��
��1����Բ����ƿ�м���m g��̼�Ͻ𣬲��������Ũ���ᣬδ��ȼ�ƾ���ǰ��A��B��������������ԭ���ǣ��ٳ����£�Fe��Ũ�����жۻ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ʾ����ú�м�������ʯ��ʯ�ۿ�ʹú����S��ȼ��ʱ������SO2ת���ɹ���CaSO4 ���Ӷ�����SO2�ŷţ��÷�������Ϊ�ƻ�������һ������ͨ������������Ӧʵ�ֵģ�
�� CaCO3 CaO+CO2 �� 2CaO+2SO2+O2 =2CaSO4
CaO+CO2 �� 2CaO+2SO2+O2 =2CaSO4
�Դˣ�ijʵ��С����������ʵ��װ�ú�ҩƷ��������Խ�����ģ��̽����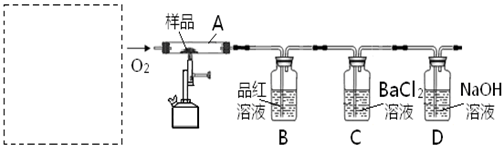
��֪ʵ��������Ʒ����---S����CaCO3��ĩ�Ļ����
��---CaSO4��ĩ
��1��ʵ��һ����ȡ��Ʒ��װ��A�С�����ͨ�����O2��������500�����ң���ַ�Ӧ���֣�B�к�ɫ��ʧ��C�г��ִ�����ɫ���ǡ�ֹͣ�ò�ʵ�����ȷ������ ��
��ȡ��Ӧ��D����Һ�μӹ������ᣬ�����������ɴ˵�֪ʵ��һ�����У� A�з�����Ӧ�Ļ�ѧ����ʽ�� ��C�з�Ӧ�����ӷ���ʽ�� ��
��Ҫ���������ʵ����ʵ��һ�µĽ��ۣ������B��Ʒ����Һ���� ��
a.��̪��NH3��H2O��Һ b.����KMnO4��Һ
c.��̪��NaHCO3��Һ d.Br2��CCl4��Һ
��2��ʵ�������ȡ��Ʒ��װ��A�С�����ͨ�����O2����Ѹ�ټ������£�������1000�����ң���ַ�Ӧ������B�к�ɫ��ʧ��C�г��ְ�ɫ���ǡ�
��ȡ��Ӧ��D����Һ�μӹ������ᣬ���ִ�����ɫ���ݣ���Ӧ�����ӷ���ʽ�� ��
�۷�Ӧ������A�����¹�������ˮ�У�ȡ���ϲ���Һ���μ�BaCl2��Һ�������а�ɫ�������֡�
�ɴ˵�֪ʵ����� A�з����ķ�Ӧ�� ����ѡ ���塱�����桱����
��3��ʵ������ȡ��Ʒ��װ��A�С�����ͨ�����O2��������1200�����ң���ַ�Ӧ��B�к�ɫ��ʧ����A�з�����Ӧ�Ļ�ѧ����ʽ�� ���Ƚ�ʵ��һ����������֪�����ø÷��������еĻ��������� ��
��4������ѧ��ѧʵ�����װ��Aͨ��ֻ��ѡ Ϊ���������IJ����ܣ�������ѡ����һ�㲻�ף��������û�ѧ����ʽ�����ǣ� ��
��5��ʵ������O2�����ɿ����ṩ���Ҷ�����ʵ��̽����ʵ������Ӱ�죬Ӧ��Aǰ����һװ��ijҩƷ��װ�á��뽫��ҩƷ��װ��ͼʾ��ͼ�е����߿��ڡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ��֤����ʵ�����Ʊ�Cl2�Ĺ����л���ˮ������HCl�ӷ���������ͬѧ�������ͼ��ʾ��ʵ��װ�ã���Ҫ��ش����⣺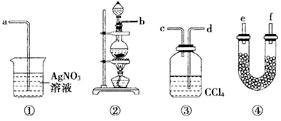
��1������ݼ�ͬѧ����ͼ��������Ӧ��װ�ã��ӿ�˳��b�� �� �� �� ��
��
��2��U�ι�����ʢ�Լ��Ļ�ѧʽΪ ��
��3��װ�â���CCl4�������� ��
��4����ͬѧ��Ϊ��ͬѧʵ����ȱ�ݣ�����֤������ͨ��AgNO3��Һ�е�����ֻ��һ�֣�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��AgNO3��Һ������ֻ��һ�֣���ͬѧ�����ij����װ��֮���ټ�װ�âݡ�����Ϊװ�â�Ӧ���� ֮�䣨��װ����ţ���ƿ�п��Է��� ��
��5����ͬѧ������ͬѧ��Ƶ�װ�ú����������װ�ã�ֻ�轫ԭ���ձ��е�AgNO3��Һ����������Һ������Ϊ�ɽ���Һ���� ������۲쵽 ������֤����Cl2ʱ��HCl�ӷ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪A��BΪ���ʣ�CΪ�����
A+B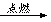 C
C
 A+B
A+B
��ʵ������ת����ϵ����
����C����ˮ��õ�ǿ����Һ����A������Na
����C����Һ��Na2CO3�ų�CO2���壬��A������H2
����C����Һ�еμ�KSCN��Һ�Ժ�ɫ����B����ΪFe
����C����Һ�еμ�NaOH��Һ����ɫ�������ɣ���B����ΪCu
A���٢� B���ڢ� C���٢� D���ۢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com