
����Ŀ����Ҫ����д��
(1) �ҹ��Ŵ��Ĵ���֮һ�ĺڻ�ҩ������Ƿۡ�����غ�ľ̿����һ��������϶��ɵģ���ըʱ�Ļ�ѧ��ӦΪ��S+2KNO3+3C=K2S+N2��+3CO2�����÷�Ӧ����������_____����ԭ����______��_____Ԫ�ر�������ÿ����6.72LCO2(��״����)������Ҫ__g��μӷ�Ӧ������˫���ŷ�����ʾ����ת�Ƶķ������Ŀ��______��
(2)�����������ʹ�õĽ���֮һ��
�ټ���![]() �����
�����![]() ��Һ�ij��÷�����_____�����߱���������_____ ��
��Һ�ij��÷�����_____�����߱���������_____ ��
�ڵ��ӹ�ҵ����![]() ��Һ��ʴ���ھ�Ե���ϵ�ͭ�����Ȼ��������Ȼ�ͭ������ӡˢ��·�壬��д��
��Һ��ʴ���ھ�Ե���ϵ�ͭ�����Ȼ��������Ȼ�ͭ������ӡˢ��·�壬��д��![]() ��Һ��ͭ��Ӧ�����ӷ���ʽ______��
��Һ��ͭ��Ӧ�����ӷ���ʽ______��
��![]() �����Ʊ�ʵ�飺
�����Ʊ�ʵ�飺
ʵ�鲽�裺ȡһ��С�ձ�������25mL����ˮ�����ձ��е�����ˮ���������ڣ����ˮ����μ���5��6��FeCl3������Һ��������С�
ʵ�������ձ�����Һ��_____ɫ����ѧ����ʽ��_____��
���𰸡�S��KNO3 C C 3.2 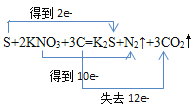 �����ЧӦ ��ɢ������ֱ����С 2Fe3++Cu==2Fe2++2Cu2+ ���ɫ FeCl3��3H2O =Fe(OH)3�����壩��3HCl
�����ЧӦ ��ɢ������ֱ����С 2Fe3++Cu==2Fe2++2Cu2+ ���ɫ FeCl3��3H2O =Fe(OH)3�����壩��3HCl
��������
��1����ӦS+2KNO3+3C�TK2S+N2��+3CO2���У�N��SԪ�ػ��ϼ۽��ͣ�����ԭ��CԪ�ػ��ϼ����ߣ����������ݴ˷�����
��2��Fe(OH)3������Ʊ��������ڷ�ˮ�еμӱ����Ȼ�����Һ����������Һ�ʺ��ɫ�����ֽ������Һ�ķ������ö����ЧӦ���������������ɢϵ�ı��������Ƿ�ɢ�ʵĿ�����С��
��1����������������N��SԪ�ػ��ϼ۽��ͣ���������S��KNO3��CԪ�ػ��ϼ����ߣ�����������ԭ����C�����ɱ�״����6.72LCO2����ʱ����0.3mol������0.1molS�μӷ�Ӧ������Ϊ3.2g��˫���ŷ���ʾ����ת�Ƶķ������Ŀ�� ���ʴ�Ϊ��S��KNO3����C��C��3.2��
���ʴ�Ϊ��S��KNO3����C��C��3.2�� ��
��
��2���ټ��������Һ�ij��÷����Ƕ����ЧӦ����ɢ����ֱ���Ĵ�С�ǽ����������ɢϵ�ı������𣬹ʴ�Ϊ�������ЧӦ����ɢ������ֱ����С��
��FeCl3��Һ��ͭ��Ӧ�����ӷ���ʽΪ2Fe3++Cu�T2Fe2++Cu2+���ʴ�Ϊ��2Fe3++Cu�T2Fe2++Cu2+��
��Fe(OH)3������Ʊ��������ڷ�ˮ�еμӱ����Ȼ�����Һ����������Һ�ʺ��ɫ����Ӧ�Ļ�ѧ����ʽΪ��FeCl3��3H2O =Fe(OH)3(����)��3HCl���ʴ�Ϊ�����ɫ��FeCl3��3H2O =Fe(OH)3(����)��3HCl��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�Ǽ״�ȼ�ϵ�صĽṹʾ��ͼ������ܷ�ӦΪ��2CH3OH+3O2��2CO2+4H2O������˵����ȷ���ǣ��� ����

A. ��缫Ϊ��صĸ�����a��ͨ��������ǿ���
B. �ҵ缫Ϊ��صĸ�����b��ͨ��������Ǽ״�
C. ������ӦʽΪ��CH3OH+H2O-6e-= CO2+6H+
D. ������ӦʽΪ��O2+2H2O -4e-= 4OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Һ��������Խǿ,�絼��Խ��������0.1000 mol/L����ֱ�ζ�10.00 mLŨ�Ⱦ�Ϊ0.1000 mol/L��NaOH��Һ�Ͷ��װ�[(CH3)2NH]��Һ{���װ���ˮ�е����백����,��֪�ڳ�����Kb[(CH3)2NH��H2O]=1.6��10-4},���ô�������õζ���������Һ�ĵ絼����ͼ��ʾ������˵����ȷ����
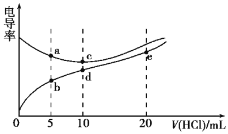
A.����ζ����װ�ʵ����ѡ���̪��ָʾ����ѡ��������С
B.d����Һ��:c(H+)>c(OH-)+c[(CH3)2NH2+]
C.b��c��d��e�ĵ����Һ��,ˮ�ĵ���̶�������d��
D.a����Һ��d�����Һ��Ϻ����Һ��: c(OH-) < c(H+) < c[(CH3)2 NH2+] <c(Na+)< c(Cl-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ͭ�����ճ������г����Ľ���,���Ź㷺����;����ش���������:
(1)ijͬѧд������ԭ�ӵ�4�ֲ�ͬ״̬�ĵ����Ų�ͼ����������͵���___________(����ĸ)��������״̬B��״̬C����ԭ�ӹ���Ϊ___________����(����䡱�����ա�)��״̬D������ij�ּ���̬�����õ����Ų�ͼ�д�����Ҫ�Dz�����__________________________��
A. ![]()
B. ![]()
C. ![]()
D. ![]()
(2)K3[Fe(CN)6]��Һ�����ڼ���Fe2+,���ɳ��������ӷ���ʽΪ______________________________����CN- ��Ϊ�ȵ�����Ļ�������______��д���ƣ���
(3)��ʢ������ͭˮ��Һ���Թ�����백ˮ,�����γ�������,�����Ӱ�ˮ,�������ܽ�,�õ�����ɫ������Һ;�����뼫�Խ�С���ܼ�(���Ҵ�),����������ɫ�ľ��塣��ͭͬһ���ڵĸ���Ԫ�صĻ�̬ԭ���У�������������ͭԭ����ͬ��Ԫ�أ���ԭ����δ�ɶԵ�����Ϊ____��ʵ��ʱ�γɵ�����ɫ��Һ�е������ӵĽṹ��ʽΪ________��SO42-�����幹��Ϊ____,����ԭ�ӵ��ӻ��������Ϊ____��

(4)ij��Al-Fe�Ͻ�ľ�����ͼ��ʾ�����Ͻ���ܶ�Ϊ��g��cm-3,����Al��Fe����С����Ϊ___ pm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ɫ����Һ�п��ܴ�������![]() ��
��![]() ��
��![]() �еļ������ӡ�
�еļ������ӡ�
(1)�����κ�ʵ��Ϳ��Կ϶�ԭ��Һ�в����ڵ�������_____��
(2)ȡ����ԭ��Һ�������ϡ���ᣬ�а�ɫ�������ɣ��ټ������ϡ���ᣬ��ɫ��������ʧ��ԭ��Һ�п϶��е�������___��
(3)ȡ(2)����Һ�������![]() �����ְ�ɫ������˵��ԭ��Һ�п϶����ڵ�������_____�����ӷ�Ӧ����ʽΪ____��
�����ְ�ɫ������˵��ԭ��Һ�п϶����ڵ�������_____�����ӷ�Ӧ����ʽΪ____��
(4)ԭ��Һ�п��ܴ������ڵ�������������![]() �е�(�����)_____��
�е�(�����)_____��
A.![]() B.
B.![]() C.
C.![]() D.
D.![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H2O2����ȡ��������ˮ���������Ӧ���ǵ�ǰ��ѧ�о����ȵ㡣 �ش��������⣺

��1��������ͬ��������������������[��NH4��2S2O8]��ԭ����ͼ��ʾ����������������Ӧ��������_______�������ĵ缫��ӦʽΪ_________��
��2��100��ʱ���ڲ�ͬ�������Ӵ����£�����������24h�ķֽ��ʼ��±���
���� | ������/��mg��L-1�� | �ֽ���/% | ���� | ������/��mg��L-1�� | �ֽ���/% | |
�� | �� | 2 | Fe3+ | 1.0 | 15 | |
Al3+ | 10 | 2 | Cu2+ | 0.1 | 86 | |
Zn2+ | 10 | 10 | Cr3+ | 0.1 | 96 |
���ϱ����ݿ�֪����ʹ��������ֽⷴӦ��ܽ�������������_______�����˹�������ʱ����ѡ�õ���������Ϊ________�����ţ���
A ���� B ��ͭ C ���� D �����
��3���������������£�H2O2��һ�ִ��ֽ�������£�
H2O2��aq����Mn2+��aq��=OH��aq����Mn3+��aq����OH����aq�� ��H��a kJ/mol
H2O2��aq����Mn3+��aq����2OH����aq��=Mn2+��aq������O2- ��aq����2H2O��l�� ����b kJ/mol
OH��aq������O2-��aq��=O2��g����OH����aq�� ��H��c kJ/mol
��2H2O2��aq��=2H2O��l����O2��g������H��_________���÷�Ӧ�Ĵ���Ϊ________��
��4��298 Kʱ����10 mL a mol��L1 NaH2PO2��10 mL 2a mol��L1 H2O2��Һ��10 mL NaOH��Һ��ϣ�������Ӧ��H2PO2-��aq����2H2O2��aq����2OH��aq��![]() PO43-��aq����4H2O��l������Һ��c��PO43-���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ��
PO43-��aq����4H2O��l������Һ��c��PO43-���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ��
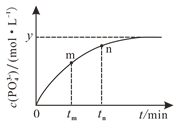
�����п��жϷ�Ӧ�ﵽƽ�����_______�����ţ���
a c��H2PO2-����y mol��L1
b ��Һ��pH���ٱ仯
c v��H2O2����2v��H2PO2-��
d c��PO43-��/c��H2PO2-�����ٱ仯
��tmʱv��_____tnʱv����������������С������������������
����ƽ��ʱ��Һ��pH��12����÷�Ӧ��ƽ�ⳣ��KΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[��ѧ��ѡ��3�����ʽṹ������]A��B��C��DΪԭ�������������������Ԫ�أ�A2����B2+������ͬ�ĵ��ӹ��ͣ�C��DΪͬ����Ԫ����C�������������������������3����DԪ���������һ��δ�ɶԵ��ӡ��ش��������⣺
��1������Ԫ���е縺�������� ����Ԫ�ط��ţ�������Cԭ�ӵĴ��������Ų�ʽΪ ��
��2������A������ͬ�������壬���зе�ߵ��� �������ʽ����ԭ���� ��B���⻯�������ľ��������� ��B�������γɵľ��壬һ������ƽ������ ��ԭ�ӡ�
��3��C��D��Ӧ��������ɱ�Ϊ1��5�Ļ�����E��E�ķ���ʽΪ ����֪�÷��ӵĿռ乹��Ϊ����˫������������Clԭ�ӱ�Fԭ��������õ��IJ���ṹ�� �֡�
��4��������D2A�����幹��Ϊ ������ԭ�ӵļ۲���Ӷ���Ϊ ������D��Na2SO3��Һ��Ӧ�������ӷ���ʽΪ ��
��5��A��B�ܹ��γɻ�����F��F�����е�B2+���ӵ����з�ʽ��ͼ��ʾ��

��ÿ��B2+��Χ����ĵȾ����B2+������ ����
����֪F�ľ���������a0=0.54nm�������ܶ�Ϊ ��ֻ��ʽ�������㣬�����ӵ�����Ϊ6.02��1023mol-1����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ����Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
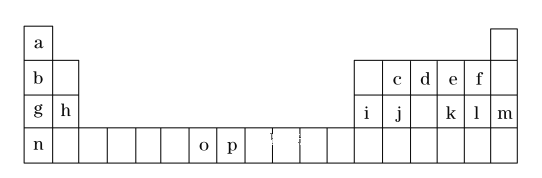
�Իش��������⣺
�ٱ��м�����ǿ��Ϊ_______(д����ѧʽ)���⻯���ȶ�����ǿ��Ϊ ______ ����д����ѧʽ��
�ڻ�̬oԭ�ӵļ۵����Ų�ͼΪ______��P3+������KSCN��Ӧ�����ɵĻ�ѧ��Ϊ _____ ����
�۽����ڱ���g��h��i��j����Ԫ�صĵ�һ�������ɴ�С����Ϊ_________ (��Ԫ�ط��ű�ʾ)��
������SiO2����(��ͼ1)������ɽ����ʯ����(��ͼ2)�е�Cԭ���û���Siԭ�ӣ�Ȼ����Si��Si֮�����Oԭ�Ӷ��γɡ�
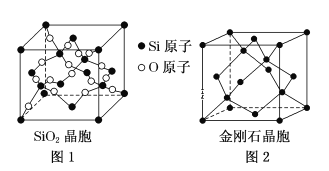
���Ʋ�SiO2������Si����______�ӻ���O��Si��O�ļ���Ϊ___________��
��һ��SiO2�����У�����_______��Siԭ�ӡ�
�۽��ʯ�;���趼��ԭ�Ӿ��壬�������ƵĽṹ�����۵㣺���ʯ_______Si���壨������������������������С������
�ܼ�����ʯ�����ı߳�Ϊa pm��NAΪ����٤���������Լ���þ������ܶ�______ g��cm��3(д������ʽ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ܺ�п����Ҫ����ɫ�������䵥�ʼ������ﱻ�㷺���ڹ��ø�������һ�ִ��л��ϴ����л����ܺ�п�Ĺ����������£�
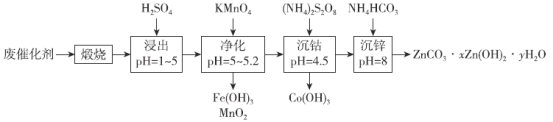
��֪��������������Һ�к���Co2����Zn2����Mn2����Fe2����Fe3���ȡ���ش�
(1)�����ա���Ŀ��Ϊ______________________________��
(2)��������ʱ����KMnO4������Ӧ���������ӷ���ʽ��_____________________________��
(3)�����ܡ�ʱ���������·�Ӧ��(NH4)2S2O8��H2O��NH4HSO4��H2O2��H2O2��H2O��O��������������������������Co3����H2O��Co(OH)3��H������ȱ�Ļ�ѧ����ʽΪ______________________________��ÿ����1 molCo(OH)3������������(NH4)2S2O8�����ʵ���Ϊ__________��
(4)Co(OH)3����Ӧ������ϡ�����ˮϴ�ӣ���������Ƿ�ϴ�Ӹɾ��ķ�����____________��
(5)�����ܡ�ʱpH����̫�ߣ���ԭ��Ϊ______________________________������п��ʱ�¶Ȳ���̫�ߣ���ԭ��Ϊ________________________________________��
(6)ȡ����п�������ù���34.1g�����պ�õ�����24.3g�������ɵ�����ͨ��������Ũ���ᣬ����5.4g�������ù���Ļ�ѧʽΪ____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com