
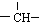 ��һ��-Cl�����Ŀ��ܽṹ�����֣���д�����ǵĽṹ��ʽ��CH3CH2CH2CHClCH3��CH3CH2CHClCH2CH3��CH3CH��CH3��CH2CH2Cl��CH2ClCH��CH3��CH2CH3��
��һ��-Cl�����Ŀ��ܽṹ�����֣���д�����ǵĽṹ��ʽ��CH3CH2CH2CHClCH3��CH3CH2CHClCH2CH3��CH3CH��CH3��CH2CH2Cl��CH2ClCH��CH3��CH2CH3�� ���� ��1������CԪ�ء�HԪ���غ�ȷ�������ķ���ʽΪC6H12���ڴ�����������H2�����ӳɷ�Ӧ������2��2-�������飬������Ľṹ��ʽΪ��CH3��3C-CH=CH2��
��2����1mol��2mol HCl��ȫ�ӳɣ������������2��˫����1��������1mol�ȴ����ܺ�4mol����������ȫȡ����Ӧ�����ȴ����������4��Hԭ�ӣ�����ԭ����������2��Hԭ�ӣ��ݴ�ȷ����
��3���ȸ���������ͨʽ����д������ʽ��Ȼ���ж�C5H11ClΪ�����һ�ȴ���жϺ���д������һ�ȴ�����칹����������²�����������ȷ��������̼���칹����������ͬ���칹�壬�ҳ���Ч����ԭ�ӣ����������ĺ����ߵ�ԭ����ԭ����һȥ������ԭ�ӣ��ݴ��жϷ���������ͬ���칹����Ŀ��
��� �⣺��1��n��������n��C����n��H��=n��������n��CO2����2n��H2O��=0.1mol��0.6mol��0.6mol��2=1��6��12����1�������к���6��Cԭ�ӡ�12��Hԭ�ӣ��ʸ����ķ���ʽΪC6H12���ڴ�����������H2�����ӳɷ�Ӧ������2.2-�������飬������Ľṹ��ʽΪ��CH3��3C-CH=CH2��
�ʴ�Ϊ����CH3��3C-CH=CH2��
��2����1mol��2mol HCl��ȫ�ӳɣ������������2��˫����1��������1mol�ȴ����ܺ�4mol����������ȫȡ����Ӧ�����ȴ����������4��Hԭ�ӣ��ȴ����������2��Hԭ���������Ȼ���ӳ�����ģ�����ԭ����������2��Hԭ�ӣ��ʸ���ΪCH��CH��
�ʴ�Ϊ��CH��CH��
��3����Է�������Ϊ72������������ͨʽΪCnH2n+2��12n+2n+2=72������õ�n=5������ʽΪ��C5H12��
�����ͬ���칹����CH3-CH2-CH2-CH2-CH3�� ��
�� ��
��
��ΪCH3-CH2-CH2-CH2-CH3��һ�ȴ����У�CH3CH2CH2CH2CH2Cl��CH3CH2CH2CHClCH3��CH3CH2CHClCH2CH3������CH3CH2CH2CHClCH3��CH3CH2CHClCH2CH3����������
��Ϊ ��һ�ȴ����У�CH3CH��CH3��CH2CH2Cl��CH3CH��CH3��CHClCH3��CH3CCl��CH3��CH2CH3��CH2ClCH��CH3��CH2CH3������CH3CH��CH3��CH2CH2Cl��CH2ClCH��CH3��CH2CH3����������
��һ�ȴ����У�CH3CH��CH3��CH2CH2Cl��CH3CH��CH3��CHClCH3��CH3CCl��CH3��CH2CH3��CH2ClCH��CH3��CH2CH3������CH3CH��CH3��CH2CH2Cl��CH2ClCH��CH3��CH2CH3����������
��Ϊ ��һ�ȴ��CH3C��CH3��2CH2Cl��������������
��һ�ȴ��CH3C��CH3��2CH2Cl��������������
�ʴ�Ϊ��CH3CH2CH2CHClCH3��CH3CH2CHClCH2CH3��CH3CH��CH3��CH2CH2Cl��CH2ClCH��CH3��CH2CH3��
���� ���⿼���л�����ƶϺ�ͬ���칹��ķ����жϣ��Ѷ��еȣ����ջ����ǽ���ؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cl2 | B�� | H+ | C�� | Cl- | D�� | HClO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������Ƶ���ˮʱ����Һ��c��H+�������� | |
| B�� | ��CO��g��+NO2��g��?CO2��g��+NO��g����ƽ����ϵ����ѹǿ��ʹ��ɫ���� | |
| C�� | �����¶��ܹ��ٽ�ˮ�ĵ��� | |
| D�� | �ں���Fe��SCN��3�ĺ�ɫ��Һ�м����ۣ����ã���Һ��ɫ��dz����ȥ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��ȥBaSO4�е�����BaCO3�����������ᣬ��ַ�Ӧ���ˡ�ϴ�ӡ����� | |
| B�� | ��ȥKCl��Һ�е�����MgCl2����������NaOH��Һ������ | |
| C�� | ��ȥCO2�����л��е�HCl���壺ͨ������̼��������Һ��ϴ�� | |
| D�� | ��ȥ��ˮ�еĵ⣬�����Ҵ���ȡ���Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ϡ��Ũ����ʱ��Ӧ������ˮ�ز���������ע��Ũ������ | |
| B�� | ������Һʱ��������ƿû�и��������������ˮ�������ƺ����ҺŨ��ƫ�� | |
| C�� | ��FeCl3��Һ�еμӹ�����ˮ������ȡFe��OH��3���� | |
| D�� | ����ij��Һ�Ƿ���SO42-ʱ��Ӧȡ��������Һ�����μ���ϡ�����BaCl2��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢۢ� | B�� | �٢ܢ� | C�� | �ۢܢ� | D�� | ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 100mL lmol/L��FeCl3��Һ��ȫ����Fe��OH��3���壬�佺����ĿΪ0.1NA | |
| B�� | 7.8 g Na2O2�к��е���������ĿΪ0.4NA | |
| C�� | 30g SiO2�����к���Si-O������ĿΪ2NA | |
| D�� | ��״����44.8L HF����ˮ�����Һ������F-����ĿΪ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K+��Ba2+��NO3-��Cl- | B�� | Ca2+��Al3+��Br-��NO3- | ||
| C�� | Na+��ClO-��SO42-��HCO3- | D�� | Mg2+��Cl-��NH4+��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 14C16O��12C18O����������������������������ԭ�Ӹ�������� | |
| B�� | 1H2��2H2��Ϊͬλ�أ����ǵ���������� | |
| C�� | 14C��14N����������ȣ�������ͬ�������� | |
| D�� | NH4+��H3O+�������ӵĵ�������������������������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com