
��1��ij�������ŷŵ���ˮ�к���Mg2+��Fe3+��Cu2+��Hg2+�������ӡ��ס��ҡ�����λѧ���ֱ�����˴Ӹ���ˮ�л��մ����Ľ���ͭ�ķ�����
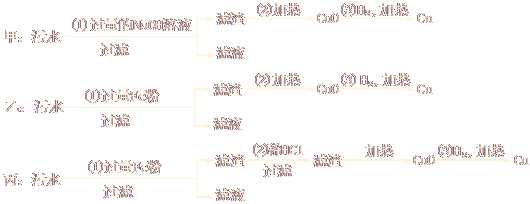
�ټס��ҡ��������ʵ�鷽���Ƿ��ܵõ������Ľ���ͭ��
��________________����_____________����________________������ܡ����ܡ���
�������Ƶ�ͭ�ķ����У���һ�������ᵼ�»�����Ⱦ��_________________��Ӧ������Щ��ʩ��ֹ��Ⱦ��__________________________��
��2����֪MgCl2��6H2O�����ڿ����м���ʱ���ͷŲ��ֽᾧˮ��ͬʱ����Mg(OH)Cl����ʽ�Ȼ�þ��������MgO�������ǹ���MgCl2��6H2O���ۺ����ã�

��ش��������⣺
����ͼ�е���������д�ʵ��ķ�Ӧ������
��Mg(OH)2������������ܽ�ƽ�⣺
Mg(OH)2(s)![]() Mg2����2OH��������ϵ�м��루���������ֲ�ͬ�������ʣ�______________��������Mg(OH)2�ܽ⡣
Mg2����2OH��������ϵ�м��루���������ֲ�ͬ�������ʣ�______________��������Mg(OH)2�ܽ⡣
��������ɫ��ѧ��ԭ�Ӿ��õĸ������ѧ��Ӧ��ԭ����ÿ��ԭ�Ӷ����뷴Ӧ��ȫ��ת��Ϊ�����203 kg MgCl2��6H2Oԭ�ϣ����Ի��29.8 kg MgO��________kg 36.5%�������________kg MgCl2��
��1���ٲ��� ���� ��
�ڱ������Ģ۲� ������������װ��
��2�����ڸ�����Ȼ��������м���
�����ࣺHCl��H2SO4��HNO3�ȣ�ǿ�������Σ�CuSO4��FeCl3�ȣ�����ࣺCH3COONH4��NH4Cl��
��149 24.2
��1���ټ�ʵ�鷽���е������ijɷ���Mg(OH)2��Fe(OH)3��Cu(OH)2��Hg(OH)2�����Ⱥ�IJ���ΪMgO��Fe2O3��CuO��HgO����H2��ԭ�õ���ͭ����MgO��Fe��Hg����ʵ�鷽���е������ijɷ���Cu��Fe��Hg�����Ⱥ�IJ���ΪCuO��Fe3O4��HgO����H2��ԭ�õ���ͭ����Fe��Hg����ʵ�鷽���м���������ۺ�������ɷ���Cu��Fe��Hg����������������ΪCu��Hg�����Ⱥ�IJ���ΪCuO����H2��ԭ�õ���ͭ�������ʡ�
�ڱ�ʵ�鷽���ܵõ������Ľ���ͭ���䲽����в����ж��Ĺ�������Ӧ������������װ�á�
��2��n(MgO)��![]() ��745 mol
��745 mol
n(MgCl2)��![]() ��745��255 mol
��745��255 mol
��m(MgCl2)��255��95��24225 g��24.225 kg
�������غ㣺36.5%������������203��29.8��24.2��149 kg��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

ͼ1-1-8
(1)����ʵ�鷽���ܷ�õ�ͭ(��ܡ����ܡ�)��
��__________����__________����__________��
(2)���Ƶ�ͭ�ķ����лᵼ�»�����Ⱦ��һ��������__________����ԭ����__________��
(3)�ڿ��Ƶ�ͭ�ķ����У���Ҫ�����ӷ���ʽΪ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣�ij�������ŷŵ���ˮ�к���Mg2+��Fe3+��Cu2+�������ӣ��ס��ҡ�����λͬѧ��ƴ���ˮ�л���ͭ�ķ������£�
��1������ʵ�鷽���ܷ�õ�ͭ����ܡ����ܡ�������_______����________��
��2���ڿ��Ƶ�ͭ�ķ����У�д�����з�Ӧ�����ӷ���ʽ_______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com