
ŃŠ¾æĪļÖŹ±ä»ÆŹ±£¬ČĖĆĒæÉŅŌ“Ó²»Ķ¬µÄ½Ē¶Č”¢²»Ķ¬µÄ²ćĆ꥓ČĻŹ¶ĪļÖŹ±ä»ÆŹ±ĖłŅżĘšµÄ»Æѧ¼ü¼°ÄÜĮæ±ä»Æ”£¾Ż“ĖÅŠ¶ĻŅŌĻĀŠšŹöÖŠ“ķĪóµÄŹĒ(””””)
A£®½šŹōÄĘÓėĀČĘų·“Ӧɜ³ÉĀČ»ÆÄĘŗó£¬Ęä½į¹¹µÄĪČ¶ØŠŌŌöĒ棬ĢåĻµµÄÄÜĮæ½µµĶ
B£®ĪļÖŹČ¼ÉÕæÉæ“×÷ŹĒ“¢“ęŌŚĪļÖŹÄŚ²æµÄÄÜĮæ(»ÆѧÄÜ)×Ŗ»ÆĪŖČČÄÜŹĶ·Å³öĄ“
C£®µŖĘų·Ö×ÓÄŚ²æ“ęŌŚ×ÅŗÜĒæµÄ»Æѧ¼ü£¬¹ŹĶس£ĒéæöĻĀµŖĘųµÄ»ÆѧŠŌÖŹŗÜ»īĘĆ
D£®ŠčŅŖ¼ÓČČ²ÅÄÜ·¢ÉśµÄ·“Ó¦²»Ņ»¶ØŹĒĪüŹÕÄÜĮæµÄ·“Ó¦

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚ298 KŹ±£¬ŹµŃé²āµĆČÜŅŗÖŠµÄ·“Ó¦£ŗH2O2+2HI====2H2O+I2£¬ŌŚ²»Ķ¬ÅØ¶ČŹ±µÄ»Æѧ·“Ó¦ĖŁĀŹ¼ūĻĀ±ķ£¬ÓÉ“ĖæÉĶĘÖŖµ±c(HI)=0.500 mol”¤L-1£¬c(H2O2)=0.400 mol”¤L-1Ź±µÄ·“Ó¦ĖŁĀŹĪŖ( )
| ŹµŃ鱹ŗÅ | 1 | 2 | 3 | 4 | 5 |
| c(HI)/mol”¤L-1”¤min-1 | 0.100 | 0.200 | 0.300 | 0.100 | 0.100 |
| c(H2O2)/mol”¤L-1”¤min-1 | 0.100 | 0.100 | 0.100 | 0.200 | 0.300 |
| v/mol”¤L-1”¤s-1 | 0.007 60 | 0.015 3 | 0.022 7 | 0.015 1 | 0.022 8 |
A.0.038 0 mol”¤L-1”¤s-1 B.0.152 mol”¤L-1”¤s-1
C.0.608 mol”¤L-1”¤s-1 D.0.760 mol”¤L-1”¤s-1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
µ½ÄæĒ°ĪŖÖ¹£¬ÓÉ»ÆѧÄÜ×Ŗ±äĪŖČČÄÜ»ņµēÄÜČŌČ»ŹĒČĖĄąŹ¹ÓĆ×īÖ÷ŅŖµÄÄÜŌ“”£
(1)»Æѧ·“Ó¦ÖŠ·Å³öµÄČČÄÜ(ģŹ±ä£¬¦¤H)Óė·“Ó¦ĪļŗĶÉś³ÉĪļŌŚ·“Ó¦¹ż³ĢÖŠ¶Ļ¼üŗĶŠĪ³ÉŠĀ¼ü¹ż³ĢÖŠĪüŹÕŗĶ·Å³öÄÜĮæµÄ“óŠ”ÓŠ¹Ų”£
ŅŃÖŖ£ŗH2(g)£«Cl2(g)====2HCl(g) ¦¤H£½£185 kJ”¤mol-1£¬¶ĻĮŃ1 mol H”ŖH¼üĪüŹÕµÄÄÜĮæĪŖ436 kJ£¬¶ĻĮŃ1 mol Cl”ŖCl¼üĪüŹÕµÄÄÜĮæĪŖ247 kJ£¬ŌņŠĪ³É1 mol H”ŖCl¼ü·Å³öµÄÄÜĮæĪŖ_________”£
(2)Č¼ĮĻČ¼ÉÕ½«ĘäĖłŗ¬µÄ»ÆѧÄÜ×Ŗ±äĪŖĪŅĆĒĖłŠčŅŖµÄČČÄÜ”£ŅŃÖŖ£ŗ
¢ŁCH4(g)£«2O2(g)====CO2(g)£«2H2O(l) ¦¤H£½£890.3 kJ”¤mol-1
¢ŚC(s,ŹÆÄ«)£«O2(g)====CO2(g) ¦¤H£½£393.5 kJ”¤mol£1
¢Ū2H2(g)£«O2(g)====2H2O(l) ¦¤H£½£571.6 kJ”¤mol-1
±ź×¼×“æöĻĀ22.4 LĒāĘųŗĶ¼×ĶéµÄ»ģŗĻĘųĢåŌŚ×ćĮæµÄŃõĘųÖŠ³ä·ÖČ¼ÉÕ·“Ó¦·Å³ö588.05 kJµÄČČĮ棬Ō»ģŗĻĘųĢåÖŠĒāĘųµÄÖŹĮæŹĒ__________”£øł¾ŻŅŌÉĻČżøöČČ»Æѧ·½³ĢŹ½£¬¼ĘĖćC(s,ŹÆÄ«)£«2H2(g)====CH4(g)µÄ·“Ó¦ČȦ¤HĪŖ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¶ĢÖÜĘŚŌŖĖŲA”¢B”¢CŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆČēĶ¼ĖłŹ¾”£ŅŃÖŖB”¢CĮ½ŌŖĖŲĖłŌŚ×åŹżÖ®ŗĶŹĒAŌŖĖŲ×åŹżµÄ2±¶,B”¢CĮ½ŌŖĖŲµÄŌ×ÓŠņŹżÖ®ŗĶŹĒAŌŖĖŲµÄ4±¶,ŌņA”¢B”¢CŹĒ””(””””)
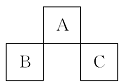
A.Be”¢Na”¢Al”””””””””””””” ”” B.B”¢Mg”¢Si
C.OӢPӢCl D.CӢAlӢP
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖH—H¼ü¼üÄÜ(¶ĻĮŃŹ±ĪüŹÕ»ņÉś³ÉŹ±ŹĶ·ÅµÄÄÜĮæ)ĪŖ436 kJ·mol£1£¬N—H¼ü¼üÄÜĪŖ391 kJ·mol£1£¬øł¾ŻČČ»Æѧ·½³ĢŹ½£ŗN2(g)£«3H2(g)===2NH3(g)””¦¤H£½£92.4 kJ·mol£1”£ŌņN”ŌN¼üµÄ¼üÄÜŹĒ(””””)
A£®431 kJ·mol£1 B£®945.6 kJ·mol£1
C£®649 kJ·mol£1 D£®896 kJ·mol£1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ(””””)
A£®ŌŚ»Æѧ·“Ó¦¹ż³ĢÖŠ£¬·¢ÉśĪļÖŹ±ä»ÆµÄĶ¬Ź±²»Ņ»¶Ø·¢ÉśÄÜĮæ±ä»Æ
B£®ĘĘ»µ·“Ó¦²śĪļČ«²æ»Æѧ¼üĖłŠčŅŖµÄÄÜĮæ“óÓŚĘĘ»µ·“Ó¦ĪļČ«²æ»Æѧ¼üĖłŠčŅŖµÄÄÜĮæŹ±£¬·“Ó¦ĪŖĪüČČ·“Ó¦
C£®·“Ó¦²śĪļµÄ×ÜģŹ“óÓŚ·“Ó¦ĪļµÄ×ÜģŹŹ±£¬·“Ó¦ĪüČČ£¬¦¤H>0
D£®¦¤HµÄ“óŠ”ÓėČČ»Æѧ·½³ĢŹ½µÄ¼ĘĮæĻµŹżĪŽ¹Ų
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ£ŗ
(1)H2(g)£«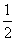 O2(g)===H2O(g) ¦¤H1£½a kJ·mol£1
O2(g)===H2O(g) ¦¤H1£½a kJ·mol£1
(2)2H2(g)£«O2(g)===2H2O(g) ¦¤H2£½b kJ·mol£1
(3)H2(g)£«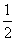 O2(g)===H2O(l) ¦¤H3£½c kJ·mol£1
O2(g)===H2O(l) ¦¤H3£½c kJ·mol£1
(4)2H2(g)£«O2(g)===2H2O(l) ¦¤H4£½d kJ·mol£1
ĻĀĮŠ¹ŲĻµŹ½ÖŠÕżČ·µÄŹĒ(””””)
A£®a<c<0 B£®b>d>0 C£®2a£½b<0 D£®2c£½d>0
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĄūÓĆČēĶ¼×°ÖĆ²ā¶ØÖŠŗĶČȵďµŃé²½ÖčČēĻĀ£ŗ
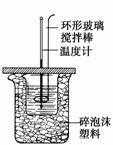
¢ŁÓĆĮæĶ²ĮæČ”50 mL 0.25 mol·L£1ĮņĖįµ¹ČėŠ”ÉÕ±ÖŠ£¬²ā³öĮņĖįĪĀ¶Č£»
¢ŚÓĆĮķŅ»ĮæĶ²ĮæČ”50 mL 0.55 mol·L£1 NaOHČÜŅŗ£¬²¢ÓĆĮķŅ»ĪĀ¶Č¼Ę²ā³öĘäĪĀ¶Č£»
¢Ū½«NaOHČÜŅŗµ¹ČėŠ”ÉÕ±ÖŠ£¬Éč·ØŹ¹Ö®»ģŗĻ¾łŌČ£¬²ā³ö»ģŗĻŅŗ×īøßĪĀ¶Č”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Š“³öĻ”ĮņĖįŗĶĻ”ĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦±ķŹ¾ÖŠŗĶČȵÄČČ»Æѧ·½³ĢŹ½(ÖŠŗĶČČŹżÖµĪŖ57.3 kJ·mol£1)£ŗ________________________________________________________________________
________________________________________________________________________ӣ
(2)µ¹ČėNaOHČÜŅŗµÄÕżČ·²Ł×÷ŹĒ______(“ÓĻĀĮŠŃ”³ö)”£
A£®ŃŲ²£Į§°ō»ŗĀżµ¹Čė B£®·ÖČż“ĪÉŁĮæµ¹Čė C£®Ņ»“ĪŃøĖŁµ¹Čė
(3)Ź¹ĮņĖįÓėNaOHČÜŅŗ»ģŗĻ¾łŌȵÄÕżČ·²Ł×÷ŹĒ______(“ÓĻĀĮŠŃ”³ö)”£
A£®ÓĆĪĀ¶Č¼ĘŠ”ŠÄ½Į°č
B£®½ŅæŖÓ²Ö½Ę¬ÓĆ²£Į§°ō½Į°č
C£®ĒįĒįµŲÕńµ“ÉÕ±
D£®ÓĆĢ×ŌŚĪĀ¶Č¼ĘÉĻµÄ»·ŠĪ²£Į§½Į°č°ōĒįĒįµŲ½Į¶Æ
(4)ŹµŃ鏿¾ŻČēĻĀ±ķ£ŗ
¢ŁĒėĢīŠ“ĻĀ±ķÖŠµÄæÕ°×£ŗ
| ĪĀ¶Č ŹµŃé“ĪŹż”” | ĘšŹ¼ĪĀ¶Čt1”ę | ÖÕÖ¹ĪĀ ¶Čt2/”ę | ĪĀ¶Č²īĘ½¾łÖµ(t2£t1) /”ę | |
| H2SO4 | NaOH | Ę½¾łÖµ | ||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 |
| 2 | 27.0 | 27.4 | 27.2 | 32.3 |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 |
¢Ś½üĖĘČĻĪŖ0.55 mol·L£1 NaOHČÜŅŗŗĶ0.25 mol·L£1ĮņĖįČÜŅŗµÄĆܶȶ¼ŹĒ1 g·cm£3£¬ÖŠŗĶŗóÉś³ÉČÜŅŗµÄ±ČČČČŻc£½4.18 J·g£1·”ę£1”£ŌņÖŠŗĶČȦ¤H£½
________________________________________________________________________
( Č”Š”ŹżµćŗóŅ»Ī»)”£
¢ŪÉĻŹöŹµŃ鏿ֵ½į¹ūÓė57.3 kJ·mol£1ÓŠĘ«²ī£¬²śÉśĘ«²īµÄŌŅņæÉÄÜŹĒ(Ģī×ÖÄø)
____________ӣ
a£®ŹµŃé×°ÖƱ£ĪĀ”¢øōČČŠ§¹ū²ī
b£®ĮæČ”NaOHČÜŅŗµÄĢå»żŹ±ŃöŹÓ¶ĮŹż
c£®·Ö¶ą“Ī°ŃNaOHČÜŅŗµ¹ČėŹ¢ÓŠĮņĖįµÄŠ”ÉÕ±ÖŠ
d£®ÓĆĪĀ¶Č¼Ę²ā¶ØNaOHČÜŅŗĘšŹ¼ĪĀ¶ČŗóÖ±½Ó²ā¶ØH2SO4ČÜŅŗµÄĪĀ¶Č
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŵ±“¶ū»Æѧ½±»ńµĆÕߏ©ĀŽæĖµČČĖ·¢ĻÖ£¬½šŹōīāµÄæرö»ÆŗĻĪļæÉŅŌ×÷ĪŖ·Ē³£ÓŠŠ§µÄĻ©Ģžø“·Ö½ā“߻ƼĮ”£¹¤ŅµÉĻŅ±Į¶īāµÄ»ÆѧŌĄķĪŖ
¢Ł2MoS2+7O2====2MoO3+4SO2£»
¢ŚMoO3+2NH3”¤H2O====(NH4)2MoO4+H2O£»
¢Ū(NH4)2MoO4+2HCl====H2MoO4”ż+2NH4Cl£»
¢ÜH2MoO4 MoO3+H2O£»
MoO3+H2O£»
¢ŻÓĆ»¹Ō¼Į½«MoO3»¹Ō³É½šŹōīā”£
ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
A.·“Ó¦¢ŁŗĶ¢Ż¶¼ŹōÓŚÖĆ»»·“Ó¦
B.½ųŠŠ¢Ś¢Ū¢Ü²½·“Ó¦µÄÄæµÄŹĒĢį“æMoO3
C.ŌŚ·“Ó¦¢ŁÖŠMoŗĶS¾ł±»Ńõ»Æ
D.ĄūÓĆH2”¢COŗĶĀĮ·Ö±š»¹ŌµČĮæµÄMoO3£¬ĻūŗÄ»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ2”Ć2”Ć3
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com