
 ��1�������й�������ʹ�ã���ʵ�ֵ���
��1�������й�������ʹ�ã���ʵ�ֵ���| (V2-V1)a |
| V |
| (V2-V1)a |
| V |
| (V2-V1)a |
| V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��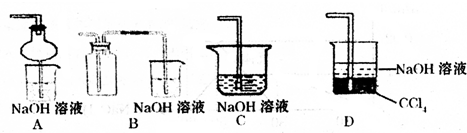
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������ƽ��ȡ11.70gʳ�� | B������Ͳ��ȡ12.36mL���� | C������ʽ�ζ�����ȡ21.20mL 0.10mol/L H2SO4��Һ | D���ù㷺pH��ֽ�ⶨpH=9.1����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
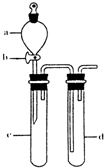
 ��1�������й�������ʹ�ã���ʵ�ֵ���______������ţ�
��1�������й�������ʹ�ã���ʵ�ֵ���______������ţ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ����һ�и߶����£����л�ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com