



 Š”ѧ¶į¹ŚAB¾ķĻµĮŠ“š°ø
Š”ѧ¶į¹ŚAB¾ķĻµĮŠ“š°ø ABCæ¼ĶõČ«ÓžķĻµĮŠ“š°ø
ABCæ¼ĶõČ«ÓžķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¹Ū²ģĖįŹ½µĪ¶Ø¹ÜŅŗĆꏱ£¬æŖŹ¼ø©ŹÓ£¬µĪ¶ØÖÕµćĘ½ŹÓ£¬Ėł²ā³öµÄ¼īŅŗµÄÅضČÖµ |
| B£®ÓƱź×¼ŃĪĖįµĪ¶ØĪ“ÖŖÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗŹ±£¬ĖįŹ½µĪ¶Ø¹ÜĻ“¾»ŗó£¬Ć»ÓŠÓƱź×¼ŃĪĖįČóĻ“¶ųÖ±½Ó×°±ź×¼ŃĪĖįµĪ¶Ø¼īŅŗ£¬Ėł²ā³öµÄ¼īŅŗµÄÅضČÖµ |
| C£®ÓĆŅŃÖŖÅØ¶ČµÄŃĪĖįČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗÓĆ·ÓĢŖ×öÖøŹ¾¼ĮĖł²ā³öµÄ¼īŅŗµÄÅضČÖµ |
| D£®×öÖŠŗĶČČ²ā¶ØŹ±£¬ŌŚ“óŠ”Į½øöÉÕ±Ö®¼äƻӊµęĖéÅŻÄĖÜĮĻ(»ņÖ½Ģõ)Ėł²ā³öµÄÖŠŗĶČČŹżÖµ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
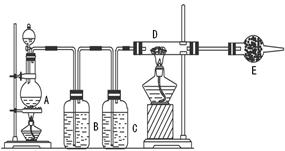
 2CaSO4+2Cl2”ü+2H2O ”£ĖūĆĒÉč¼ĘĮĖČēĻĀÖĘČ”ĀČĘų²¢ŃéÖ¤ĘäŠŌÖŹµÄŹµŃ锣
2CaSO4+2Cl2”ü+2H2O ”£ĖūĆĒÉč¼ĘĮĖČēĻĀÖĘČ”ĀČĘų²¢ŃéÖ¤ĘäŠŌÖŹµÄŹµŃ锣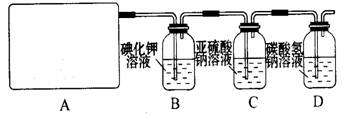
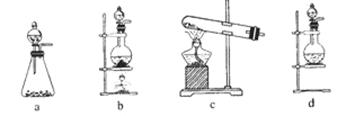
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
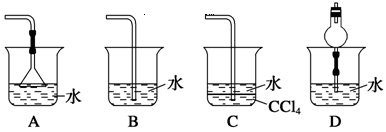
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¢Ś¢Ž¢Ū¢ß¢Ż | B£®¢Ü¢Ū¢Ż¢ß¢Ž | C£®¢Ł¢Ū¢ß¢Ż¢Ž | D£®¢Ś¢Ż¢ß¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
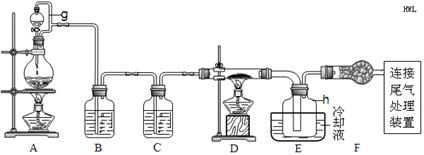
| ĪļÖŹ | SiCl4 | BCl3 | AlCl3 | FeCl3 | PCl5 |
| ·Šµć/”ę | 57.7 | 12.8 | ”Ŗ | 315 | ”Ŗ |
| ČŪµć/”ę | £70.0 | £107.2 | ”Ŗ | ”Ŗ | ”Ŗ |
| Éż»ŖĪĀ¶Č/”ę | ”Ŗ | ”Ŗ | 180 | 300 | 162 |
 5Fe3£«£«Mn2£«£«4H2O
5Fe3£«£«Mn2£«£«4H2O²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¢Ł¢Ś¢Ū¢Ü¢Ż | B£®¢Ś¢Ü¢Ż¢Ū¢Ł | C£®¢Ü¢Ś¢Ū¢Ł¢Ż | D£®¢Ś¢Ü¢Ł¢Ż¢Ū |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com