
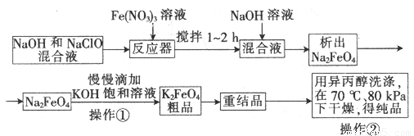
��12�֣����������һ�ָ�Ч�Ķ�ܵ�ˮ����������ҵ�ϳ�����NaClO������������ԭ��Ϊ��3NaClO + 2Fe(NO3)3+ 10NaOH��2Na2FeO4��+ 3NaCl + 6NaNO3 + 5H2O
Na2FeO4��2KOH��K2FeO4��2NaOH��Ҫ�������������£�
��1��д����Ӧ�ٵ����ӷ���ʽ ��
��2������ͼ�С�ת��������ij�����½��еģ�˵�����¶���Ksp(K2FeO4) Ksp(Na2FeO4)�������������������
��3����Ӧ���¶ȡ�ԭ�ϵ�Ũ�Ⱥ���ȶԸ�����صIJ��ʶ���Ӱ�졣
����ͼΪ��ͬ���¶��£�Fe(NO3)3��ͬ����Ũ�ȶ�K2FeO4�����ʵ�Ӱ�죻
��ͼΪһ���¶��£�Fe(NO3)3����Ũ�����ʱ��NaClOŨ�ȶ�K2FeO4�����ʵ�Ӱ�졣
�ٹ�ҵ����������¶�Ϊ �� �棬��ʱFe(NO3)3��NaClO������Һ�������Ũ��֮��Ϊ ��
����NaClO������������������л�����Fe(OH)3��д���÷�Ӧ�����ӷ���ʽ��
��
��Fe(NO3)3����������ڼ��Խ�����K2FeO4��Fe3+����������ԭ��Ӧ����K3FeO4���˷�Ӧ�����ӷ���ʽ�� ��
��4��K2FeO4��ˮ��Һ����ˮ�⣺4FeO42��+10H2O4Fe(OH)3+8OH��+3O2�����ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ��Һ������ţ���
A��H2O B��CH3COONa������� C��NH4Cl������� D��Fe(NO3)3�������
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ�ĵ��и�����ѧ������ͳ�����ۻ�ѧ�Ծ���B���������棩 ���ͣ������
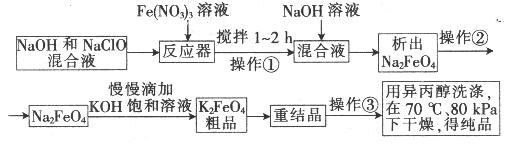
���������һ�ָ�Ч�Ķ��ˮ��������������������������������������ɱ�������������ã�������صĿ�����������������ʾ���������Ӧ��ǰ�������Ʊ��������£�
�Իش���������
��1����Ӧ���У�NaOH��NaClO��Fe(NO3)3������Ӧ����Na2FeO4����ɲ���ƽ�������ӷ�Ӧ����ʽ��
��Fe3++��ClO��+��OH�� ==��FeO42��+��Cl��+��___________
��2����������Ũ�ȶԸ�����ز�����һ��Ӱ�죬��NaClOŨ��Ϊ298g/Lʱ���������ƵIJ�����ߣ���ʱNaClO�����ʵ���Ũ��Ϊ_______________��
��3������Ϊ�������ܹ�����ת����ԭ����__________�������μӵ�ԭ�����___________��
��4������������¶ȹ�����ɸ�����صķֽ⣬����������ȷֽ�ʱ���ɽ�����������������÷�Ӧ�Ļ�ѧ����ʽΪ__________________��
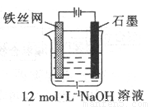
��5�������õ绯ѧ����ȡNa2FeO4����װ����ͼ��ʾ���������ĵ缫��ӦʽΪ ��
�����ĵ缫��ӦʽΪ______________�����һ��ʱ�����OH����Ũ�Ȼ�_________(����ߡ��������͡�
���䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ����12�½��Բ��Ի�ѧ�Ծ��������棩 ���ͣ������
���������һ�ָ�Ч�Ķ��ˮ��������������������������������������ɱ�������������ã�������صĿ�����������������ʾ���������Ӧ��ǰ�������Ʊ�·�����£�

�Իش���������
(1)�����ٺ�Ļ��Һ�м���NaOH��Һ�������ǣ� ��
����������ϴ�Ӳ�Ʒ��Ŀ���� ��
(2) ���������������ͬ���ò��������ǣ�___________________��
(3)��Ӧ���У�NaOH��NaClO��Fe(NO3)3��Ӧ�����ӷ���ʽΪ�� _____________________��
(4)��������Ũ�ȶԸ�����ز�����һ��Ӱ�죨��ͼ�ף�����������ز������ʱ��NaClO�����ʵ���Ũ��ԼΪ_________��������������һλС����

(5)�ӻ��������ĽǶȿ����Ʊ��м����Na2FeO4�Ϻõķ���Ϊ�绯ѧ������װ����ͼ����ʾ���������ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ������ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ʵ����
���������һ�ָ�Ч�Ķ��ˮ��������������������������������������ɱ�������������ã�������صĿ�����������������ʾ���������Ӧ��ǰ�������Ʊ�·�����£�
�Իش���������
��1�������ٽ����Ŀ���ǣ� ��
��2�����������������ͬ���ò��������ǣ�___________________��
��3����Ӧ���У� NaOH��NaClO��Fe(NO3)3��Ӧ�����ӷ���ʽΪ�� _______________________��
��4����������Ũ�ȶԸ�����ز�����һ��Ӱ�죬��������ز������ʱ��NaClO�����ʵ���Ũ��ԼΪ_________��������������һλС����

��5���ӻ��������ĽǶȿ����Ʊ��м����Na2FeO4�Ϻõķ���Ϊ�绯ѧ������װ����ͼ����ʾ���������ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�����������и������в��ԣ�һ����ѧ�Ծ� ���ͣ������
��12�֣����������һ�ָ�Ч�Ķ�ܵ�ˮ����������ҵ�ϳ�����NaClO������������ԭ��Ϊ��
3NaClO + 2Fe(NO3)3 + 10NaOH��2Na2FeO4��+ 3NaCl + 6NaNO3 + 5H2O
Na2FeO4��2KOH��K2FeO4��2NaOH
��Ҫ�������������£�
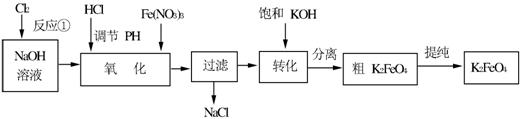
��1��д����Ӧ�ٵ����ӷ���ʽ_____________________________��
��2������ͼ�С�ת��������ij�����½��еģ�˵�����¶���Ksp(K2FeO4) _________ Ksp(Na2FeO4)�������������������
��3����Ӧ���¶ȡ�ԭ�ϵ�Ũ�Ⱥ���ȶԸ�����صIJ��ʶ���Ӱ�졣
ͼ1Ϊ��ͬ���¶��£�Fe(NO3)3��ͬ����Ũ�ȶ�K2FeO4�����ʵ�Ӱ�죻
ͼ2Ϊһ���¶��£�Fe(NO3)3����Ũ�����ʱ��NaClOŨ�ȶ�K2FeO4�����ʵ�Ӱ�졣

�ٹ�ҵ����������¶�Ϊ_______�棬��ʱFe(NO3)3��NaClO������Һ�������Ũ��֮��Ϊ_______��
����NaClO������������������л�����Fe(OH)3��д���÷�Ӧ�����ӷ���ʽ��
____________________________________________________��
��Fe(NO3)3����������ڼ��Խ�����K2FeO4��Fe3+����������ԭ��Ӧ����K3FeO4���˷�Ӧ�����ӷ���ʽ��____________________________________��
��4��K2FeO4 ��ˮ��Һ����ˮ�⣺4FeO42��+10H2O 4Fe(OH)3+8OH��+3O2�����ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��_______��Һ������ţ���
4Fe(OH)3+8OH��+3O2�����ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��_______��Һ������ţ���
A��H2O B��CH3COONa������� C��NH4Cl������� D��Fe(NO3)3�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com