
| |||||||||||||||

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĖÄ“ØŹ”½šĢĆ֊ѧ2011£2012ѧğø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗ058
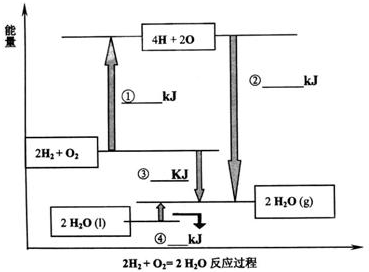
»Æѧ¼üµÄ¼üÄÜŹĒÖøĘųĢ¬Ō×Ó¼äŠĪ³É1 mol»Æѧ¼üŹ±ŹĶ·ÅµÄÄÜĮ森Čē£ŗH(g)£«I(g)”śH£I(g)£«297 KJ¼“H£I¼üµÄ¼üÄÜĪŖ297 kJ/mol£¬Ņ²æÉŅŌĄķ½āĪŖĘĘ»µ1 mol””H£I¼üŠčŅŖĪüŹÕ297 KJµÄČČĮ森»Æѧ·“Ó¦µÄ·¢ÉśæÉŅŌ擳ɾɻÆѧ¼üµÄĘĘ»µŗĶŠĀ»Æѧ¼üµÄŠĪ³É£®ĻĀ±ķŹĒŅ»Š©¼üÄÜŹż¾Ż£®(µ„Ī»£ŗkJ/mol)
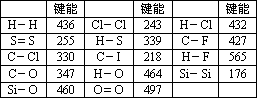
ŌĶĮÉĻŹöŠÅĻ¢£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)øł¾Ż±ķÖŠŹż¾ŻÅŠ¶ĻCCl4µÄĪČ¶ØŠŌ________(Ģī”°“óÓŚ”±»ņ”°Š”ÓŚ”±)CF4µÄĪČ¶ØŠŌ£®ŹŌŌ¤²āC£Br¼üµÄ¼üÄÜ·¶Ī§________£¼C£Br¼üÄÜ£¼________
(2)ÓŠČĖČĻĪŖ£ŗH£O¼üµÄ¼üÄÜ“óÓŚH£S¼üµÄ¼üÄÜ£¬ĖłŅŌH2OµÄČŪ·ŠµćøßÓŚH2SµÄČŪ·Šµć£®ÄćŹĒ·ńŌŽĶ¬ÕāÖÖ¹Ūµć£æČē²»ŌŽĶ¬£¬ĒėĖµ³öÄćµÄ½āŹĶ£®________£®
(3)ŅŃÖŖH2O(l)£½H2O(g)””¦¤H£½£«44 kJ/mol£¬ĒėŠ“³ö±ķŹ¾ĒāĘųČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½£ŗ________£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äźø߶ž”°ĆæÖÜŅ»Į·”±ĻµĮŠ(19)»ÆѧŹŌĢā ĢāŠĶ£ŗ022
»Æѧ¼üµÄ¼üÄÜŹĒÖøĘųĢ¬Ō×Ó¼äŠĪ³É1 mol»Æѧ¼üŹ±ŹĶ·ÅµÄÄÜĮ森Čē£ŗH(g)£«I(g)”śH£I(g)£«297 KJ£¬¼“H£I¼üµÄ¼üÄÜĪŖ297 kJ/mol£¬Ņ²æÉŅŌĄķ½āĪŖĘĘ»µ1 mol””H£I¼üŠčŅŖĪüŹÕ297 KJµÄČČĮ森»Æѧ·“Ó¦µÄ·¢ÉśæÉŅŌ擳ɾɻÆѧ¼üµÄĘĘ»µŗĶŠĀ»Æѧ¼üµÄŠĪ³É£®ĻĀ±ķŹĒŅ»Š©¼üÄÜŹż¾Ż£®(µ„Ī»£ŗkJ/mol)
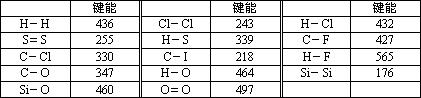
ŌĶĮÉĻŹöŠÅĻ¢£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)øł¾Ż±ķÖŠŹż¾ŻÅŠ¶ĻCCl4µÄĪČ¶ØŠŌ________(Ģī”°“óÓŚ”±»ņ”°Š”ÓŚ”±)CF4µÄĪČ¶ØŠŌ£®ŹŌŌ¤²āC£Br¼üµÄ¼üÄÜ·¶Ī§________£¼C£Br¼üÄÜ£¼________£®
(2)½įŗĻ±ķÖŠŹż¾ŻŗĶČČ»Æѧ·½³ĢŹ½H2(g)£«Cl2(g)£½2HCl(g)””¦¤H£½£QKJ/ mol£»Ķعż¼ĘĖćČ·¶ØČČ»Æѧ·½³ĢŹ½ÖŠQµÄÖµĪŖ________
(3)ÓŠČĖČĻĪŖ£ŗH£O¼üµÄ¼üÄÜ“óÓŚH£S¼üµÄ¼üÄÜ£¬ĖłŅŌH2OµÄČŪ·ŠµćøßÓŚH2SµÄČŪ·Šµć£®ÄćŹĒ·ńŌŽĶ¬ÕāÖÖ¹Ūµć£æČē²»ŌŽĶ¬£¬ĒėĖµ³öÄćµÄ½āŹĶ£®________________
(4)¢ŁSi£Si¼üÄÜŠ”ÓŚSi£O¼üÄܵĥķÓÉŹĒ£ŗ________________
¢ŚĒėŠ“³ö¾§Ģå¹čÓėŃõĘų·“Ӧɜ³É¶žŃõ»Æ¹čµÄČČ»Æѧ·½³ĢŹ½£ŗ________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
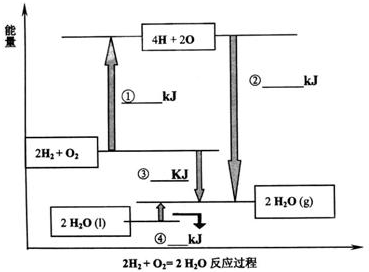
| A£®¢Ł¹ż³ĢĪüŹÕÄÜĮæĪŖQ1+Q2 |
| B£®¢Ś¹ż³Ģ·Å³öÄÜĮæĪŖ2Q3 |
| C£®¢Ū¹ż³ĢÄÜĮæ±ä»ÆĪŖ4Q3-£Ø2Q1+Q2£© |
| D£®¢ÜĪüŹÕÄÜĮæĪŖ4Q3 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com